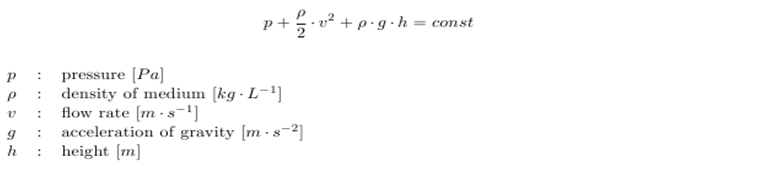Team:Wageningen UR/Project/DevicesSetup
From 2011.igem.org
(→Custom fluidic device designed by Team Wageningen UR to measure oscillations) |
(→Custom fluidic device designed by Team Wageningen UR to measure oscillations) |
||
| Line 24: | Line 24: | ||
=== Setup === | === Setup === | ||
| - | + | <!-- | |
In order to physically constrain the bacteria, Hasty used a trapping chamber as depicted in Figure Xsome below. The chamber had the dimensions of 1X1 micron. This limited the cell growth to forming a monolayer (sentence). Excess cells and AHL were flushed away through the main chanel. [REF] | In order to physically constrain the bacteria, Hasty used a trapping chamber as depicted in Figure Xsome below. The chamber had the dimensions of 1X1 micron. This limited the cell growth to forming a monolayer (sentence). Excess cells and AHL were flushed away through the main chanel. [REF] | ||
| - | [[File:Hasty_device_WUR.png| | + | [[File:Hasty_device_WUR.png|200px|center]] [ref][[File:Legend_device_WUR.png|140px]] |
| - | + | ||
| - | [[File:Legend_device_WUR.png| | + | |
To create a monolayer, the micro-sieve seemed promising. The cells could be drawn toward the sieve by manually applying an under pressure with a syringe. Since the Top10 ''e.coli'' strain used for our transformations does not form biofilms, additional cells would be flushed away once all the pores of the micro-sieve are blocked. However the resulting flow over this monolayer turned out to be in the wrong dimensional plane (how to say this?). The direction of the flow over the micro-sieve can be seen in figure Xsome+1. | To create a monolayer, the micro-sieve seemed promising. The cells could be drawn toward the sieve by manually applying an under pressure with a syringe. Since the Top10 ''e.coli'' strain used for our transformations does not form biofilms, additional cells would be flushed away once all the pores of the micro-sieve are blocked. However the resulting flow over this monolayer turned out to be in the wrong dimensional plane (how to say this?). The direction of the flow over the micro-sieve can be seen in figure Xsome+1. | ||
| - | [[File:Micro-sieve_device_WUR.png| | + | [[File:Micro-sieve_device_WUR.png|270px|center]] |
The setup as shown above imposes the problem that the diffusion of AHL is much slower than the flow rate over the sieve. Therefore the AHL produced will always be flushed away before a uniform concentration can be established over the whole cell culture, thus preventing any synchronized behaviour to arise. The use of a micro-sieve in the course of our iGEM project was therefore discarded. | The setup as shown above imposes the problem that the diffusion of AHL is much slower than the flow rate over the sieve. Therefore the AHL produced will always be flushed away before a uniform concentration can be established over the whole cell culture, thus preventing any synchronized behaviour to arise. The use of a micro-sieve in the course of our iGEM project was therefore discarded. | ||
| Line 41: | Line 39: | ||
| - | [[File:Micro-dish1_device_WUR.png| | + | [[File:Micro-dish1_device_WUR.png|290px]] [[File:Flushingoutofwells_WUR.gif|300px|right]] |
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | Since flowing over the wells(howtowritethis.) it's not necessary, so in the end only bottom flow was used. as seen in the picture below. As a result, the bacteria were bottom fed as seen in the scheme in figure Xsomeplussomemore. This allowed the measurements to be taken continuously for various hours, as nutrients can diffuse through the bottom of the well. | ||
| + | |||
| + | [[File:Micro-dish2_device_WUR.png|200px]] [[File:Bottom_feed_WUR.png|500px|right]] | ||
| + | |||
| Line 47: | Line 56: | ||
| - | |||
| - | |||
| - | |||
'''Fig.Xwhatever:''' ''Applying bottom feeding to keep the cells in the wells alive'' | '''Fig.Xwhatever:''' ''Applying bottom feeding to keep the cells in the wells alive'' | ||
| Line 57: | Line 63: | ||
| + | [[Team:Wageningen_UR/Project/DevicesSetup#Customary_fluidic_device_designed_by_Team_Wageningen_UR_to_measure_oscillations| back to top]] | ||
| - | |||
| + | --> | ||
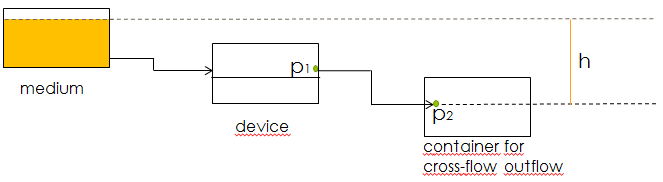
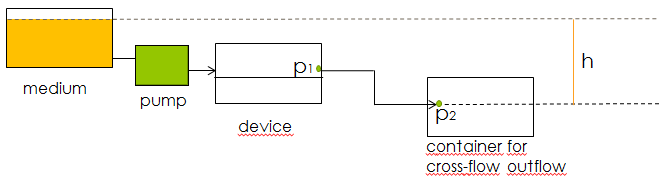
Another concern for the setup of the device was to be able to gain control over the flow rate. According to Bernoulli's principle, the velocity of a fluid can be influenced by varying the height of the medium bottle. This approach was also used in the paper cited above. Figure X. shows the corresponding setup and the applying equations. | Another concern for the setup of the device was to be able to gain control over the flow rate. According to Bernoulli's principle, the velocity of a fluid can be influenced by varying the height of the medium bottle. This approach was also used in the paper cited above. Figure X. shows the corresponding setup and the applying equations. | ||
| Line 75: | Line 82: | ||
'''Fig.X+1:''' ''Setup of the device using a pump to control the velocity of the fluid.'' | '''Fig.X+1:''' ''Setup of the device using a pump to control the velocity of the fluid.'' | ||
| + | |||
| Line 80: | Line 88: | ||
| - | [[File:Closeup_device_WUR.JPG| | + | [[File:Closeup_device_WUR.JPG|200px]] [[File:Setup_WUR.jpg|500px|right]] |
| Line 96: | Line 104: | ||
| - | [[Team:Wageningen_UR/Project/DevicesSetup# | + | [[Team:Wageningen_UR/Project/DevicesSetup#Custom_fluidic_device_designed_by_Team_Wageningen_UR_to_measure_oscillations| back to top]] |
}} | }} | ||
Revision as of 18:46, 19 September 2011
 "
"