Team:UT-Tokyo/Data/Modeling/Model01
From 2011.igem.org
| Line 2: | Line 2: | ||
=Aim= | =Aim= | ||
| - | We | + | We needed to know the behavior of L-Asp diffusion to perform our entire simulation (model3). |
| - | to | + | We experimentaly researched Asp diffusion using TLC method but the results were insufficient for the entire simulation. |
| + | So we decided to investigate the Asp diffusion by numerical simulation. | ||
=Method= | =Method= | ||
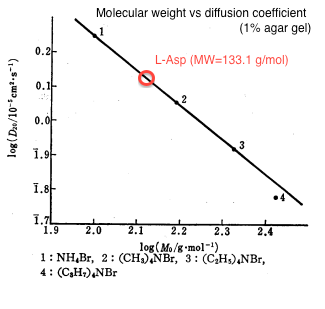
| - | We first estimated the value of diffusion coefficient by interpolating molecular mass of L-Asp (133)<html><sup class="ref">[1]</sup></html> in figure 1. | + | We first estimated the value of diffusion coefficient by interpolating molecular mass of L-Asp (133) from the relationship between molecular weight and diffusion constant<html><sup class="ref">[1]</sup></html> as shown in figure 1. |
| - | {{:Team:UT-Tokyo/Templates/Image|file= | + | {{:Team:UT-Tokyo/Templates/Image|file=utt_m1_fig1.png|caption=Figure 1. Molecular Weight v.s. Diffusion Constant}} |
The estimated value was D = 0.001 [<html>mm<sup>2</sup>/sec</html>]. | The estimated value was D = 0.001 [<html>mm<sup>2</sup>/sec</html>]. | ||
| - | Then we verify the value by | + | Then we tried to verify the value was correct by showing we could predict the experimental result theoretically using this value as a diffusion constant. |
| + | |||
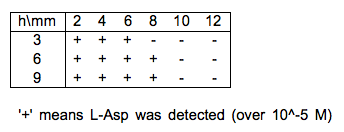
| + | Experimental data of L-Asp diffusion are shown in figure 2. | ||
| + | This is the result of TLC experiment. We dropped 2×10<html><sup>-7</sup></html> mol L-Asp on the center of agar gel at the beginning. | ||
| + | Horizontal rows indicates elapssed time and vertical columns indicates the distance from the center. | ||
| + | |||
| + | {{:Team:UT-Tokyo/Templates/Image|file=utt_m1_fig2.png|caption=Figure 2.The Result of TLC Experiment}} | ||
| + | |||
We simulated the time development of the L-Asp concentration distribution. | We simulated the time development of the L-Asp concentration distribution. | ||
| - | We solved the diffusion equation | + | We solved the diffusion equation using 1st order finite difference method. |
| - | [[File: | + | [[File:utt_m1_eqn1.png]] |
| - | + | A indicates the L-Asp concentration and D means the diffusion coefficient. | |
The shape of system was a circle with radius 5cm. | The shape of system was a circle with radius 5cm. | ||
| - | We dropped <html> | + | We dropped 2×10<html><sup>-7</sup></html> mol Asp at the center of the circle as the initial state. |
=Result= | =Result= | ||
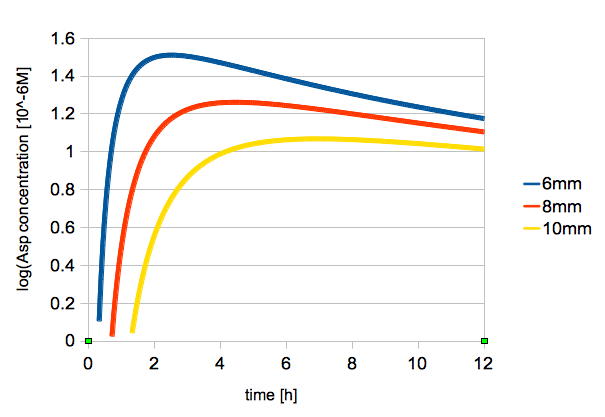
| - | {{:Team:UT-Tokyo/Templates/Image|file= | + | {{:Team:UT-Tokyo/Templates/Image|file=utt_m1_fig3.png|caption=Figure 3. Change in L-Asp Concentration Over Time (2, 6, 8 mm from the center)}} |
| - | The time change of logarithmic values of L-Asp concentration is shown in figure | + | The time change of logarithmic values of L-Asp concentration at 2, 6, 8 mm from the center is shown in figure 3. |
| - | + | ||
=Discussion= | =Discussion= | ||
| - | + | The result of the simulation shown in fig. 3 were similar to the result of experiment shown in fig. 2. | |
| - | + | So we judged that the diffusion coefficient D=0.001 was adequate value. | |
| - | + | ||
| - | + | ||
<html> | <html> | ||
| Line 37: | Line 42: | ||
<div id="references"> | <div id="references"> | ||
<ul> | <ul> | ||
| - | <li id="ref_1">[1] Toshiko M, Masayuki N. | + | <li id="ref_1">[1] Toshiko M, Masayuki N "measurement of diffusion coefficient using agar. gel" Chemical Society of Japan (1978) 26 5 p.377</li> |
</ul> | </ul> | ||
</div> | </div> | ||
</html> | </html> | ||
{{:Team:UT-Tokyo/Templates/EndContent}} | {{:Team:UT-Tokyo/Templates/EndContent}} | ||
Revision as of 13:30, 5 October 2011
Model1: L-Asp diffusion

iGEM UT-Tokyo
Aim
We needed to know the behavior of L-Asp diffusion to perform our entire simulation (model3). We experimentaly researched Asp diffusion using TLC method but the results were insufficient for the entire simulation. So we decided to investigate the Asp diffusion by numerical simulation.
Method
We first estimated the value of diffusion coefficient by interpolating molecular mass of L-Asp (133) from the relationship between molecular weight and diffusion constant[1] as shown in figure 1.
The estimated value was D = 0.001 [mm2/sec].
Then we tried to verify the value was correct by showing we could predict the experimental result theoretically using this value as a diffusion constant.
Experimental data of L-Asp diffusion are shown in figure 2. This is the result of TLC experiment. We dropped 2×10-7 mol L-Asp on the center of agar gel at the beginning. Horizontal rows indicates elapssed time and vertical columns indicates the distance from the center.
We simulated the time development of the L-Asp concentration distribution. We solved the diffusion equation using 1st order finite difference method.
A indicates the L-Asp concentration and D means the diffusion coefficient. The shape of system was a circle with radius 5cm. We dropped 2×10-7 mol Asp at the center of the circle as the initial state.
Result
The time change of logarithmic values of L-Asp concentration at 2, 6, 8 mm from the center is shown in figure 3.
Discussion
The result of the simulation shown in fig. 3 were similar to the result of experiment shown in fig. 2. So we judged that the diffusion coefficient D=0.001 was adequate value.
References
- [1] Toshiko M, Masayuki N "measurement of diffusion coefficient using agar. gel" Chemical Society of Japan (1978) 26 5 p.377
 "
"