Team:UT-Tokyo/Data/Modeling/Model01
From 2011.igem.org
| Line 7: | Line 7: | ||
=Method= | =Method= | ||
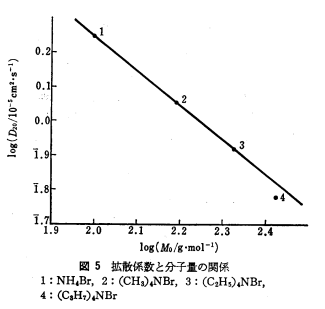
We first estimated the value of diffusion coefficient by interpolating molecular mass of L-Asp (133)<html><sup class="ref">[1]</sup></html> in figure 1. | We first estimated the value of diffusion coefficient by interpolating molecular mass of L-Asp (133)<html><sup class="ref">[1]</sup></html> in figure 1. | ||
| - | + | ||
| + | {{:Team:UT-Tokyo/Templates/Image|file=UT-Tokyo_Model01_Fig1.png|caption=Figure 1. Molecular Weight v.s. Diffusion Constant}} | ||
| + | |||
The estimated value was D = 0.001 [<html>mm<sup>2</sup>/sec</html>]. | The estimated value was D = 0.001 [<html>mm<sup>2</sup>/sec</html>]. | ||
Revision as of 14:51, 4 October 2011
Model1: L-Asp diffusion

iGEM UT-Tokyo
Aim
We determined the diffusion coefficient of L-Asp by comparing the result of numerical simulations and experiments to support the main simulation (model03).
Method
We first estimated the value of diffusion coefficient by interpolating molecular mass of L-Asp (133)[1] in figure 1.
The estimated value was D = 0.001 [mm2/sec].
Then we verify the value by comparing the result of numerical simulations and experiments. We simulated the time development of the L-Asp concentration distribution. We solved the diffusion equation
using 1st order finite difference method. The shape of system was a circle with radius 5cm. We dropped 10-2M Asp at the center of the circle as the initial state.
Result
The time change of logarithmic values of L-Asp concentration is shown in figure 2. The vertical axis indicates relative values of L-Asp concentration under condition that let its concentration where and when it is dropped to be 10,000.
Discussion
The result shows that L-Asp was detected at 8mm from the center (where the L-Asp is dropped) 3~6 hours after the samples were applied. According to our simulation, its concentration at 8mm changes earlier. However, because there are some uncertain factors such as temperature, gel condition (which change diffusion coefficient) or experimental error, we judged that the diffusion coefficient D=0.001 was correct in its order and adopted this value in model 03.
References
- [1] Toshiko M, Masayuki N. "寒天ゲルを用いる拡散係数の測定" Chemical Society of Japan (1978) 26 5 p.377.
 "
"