Team:Paris Bettencourt/tRNA diffusion
From 2011.igem.org

The tRNA amber diffusion
The amber codon and the tRNA amber suppressor
|
The amber codon is one of the less used stop codon in bacteria. The principle of the artificial amber suppresor tRNA is to provide a tRNA for the stop codon. We explain how it works in the following paragraphs When the ribosome transcripts the RNA into protein, it look for the RBS sequence, fix on it. Then, it tryes to fit with the codon it is located on with the tree bases complement of the tRNA flying round. When it finds the tRNA with the anti-codon of the start codon, with a methionine loaded on it, the translation starts. Then, codon after codon, the ribosome try to fit many tRNA on the codon it is placed on, until it find the correct one, fix the corresponding amino-acid and then and then moves one codon farther. When the ribosome doesn't find the correct tRNA for the codon it is located on, the ribosome declare this codon is a stop, and release the peptide and the mRNA. The idea behind the tRNA amber supressor is to create an artificial tRNA, based on an existing tRNA that is loaded with a specific amino-acid, and to change its anti-codon, replacing it by the amber anti-codon. By expressing this artificial tRNA in the cell, the ribosome can find a tRNA that match with the amber codon, skip the stop and keep polymerasing the protein. |
    |

Fig1: Transcription schematics animation |
By creating a protein that carries amber mutation in the middle of its coding sequence, in the place of the amino-acid that loaded on the tRNA amber supressor, we create a protein that can be properly transcribed only if the artificial tRNA amber suppressor is present in the cell. This approach is sometime used in synthetic biology to create artificial AND gates.
Surprisingly, the cells survives the expression of the amber tRNA although it is a really lethal object, because it prevents the cell from expressing properly almost 20% of her endogenic proteins.
Building a new tRNA amber supressor for B. subtilis
There were no tRNA amber supressor in the registry for B. Subtilis. Using biofinformatics analysis we found out that the tRNA sequence is quite different from E. coli to B. subtilis. So we decided to build our new one. The problem was the choice of the aminu-acid we wanted to hijack. We found that some of le loading proteins recognize the anti-codon. As we are going to modify it, we need to choose an tRNA in which the anti-codon is not strongly recognized by the loading protein.
We found out in this paper[1] that some people managed to create a tyrosine amber tRNA in B. Subtilis, so we decided to work on this amino-acid. Many question around the maturation of the mRNA into the tRNA remained unsolved so we decide to build it with and without a translation terminator.
Principle of the design
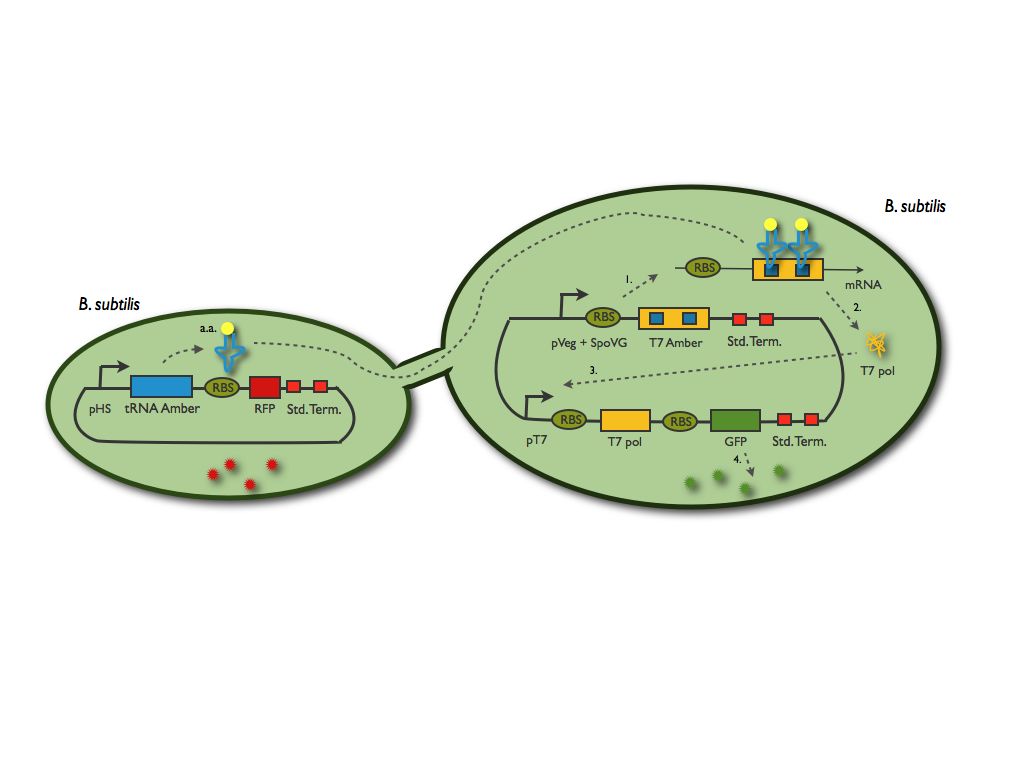
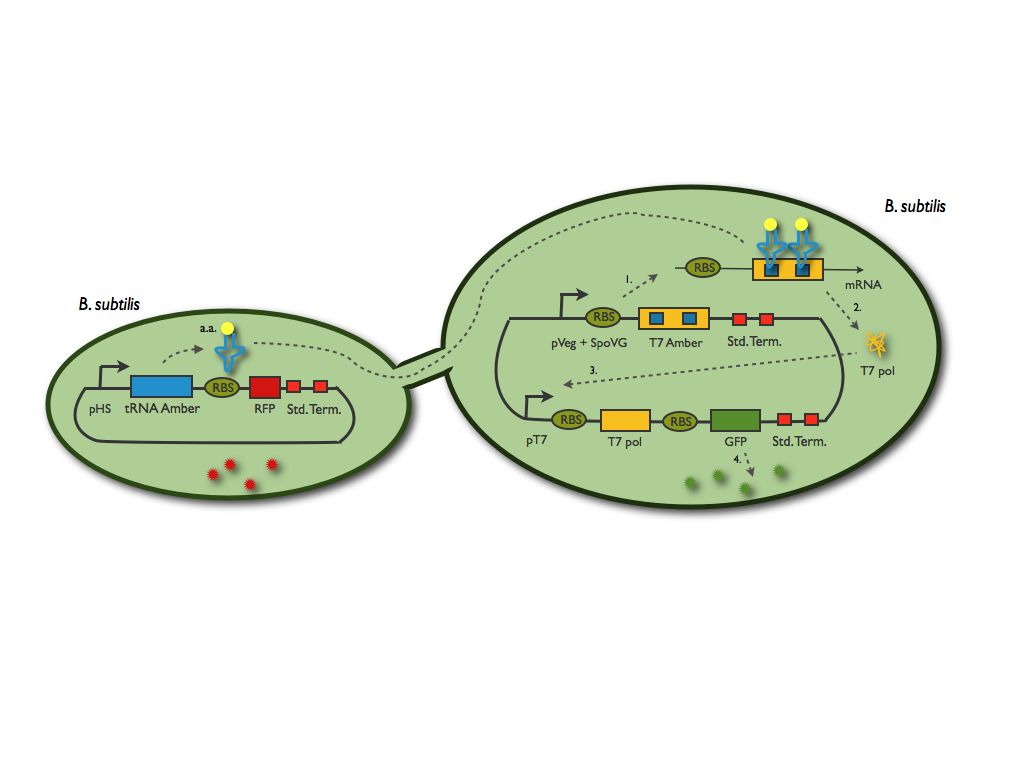
The emitter cell will produce this mutated transfer RNA (we mutated a YtRNA for it to recognise the stop codon) and the receiver cell will then be able to translate a protein (we chose the T7 polymerase) which gene contain an amber mutation. This protein will part of a reporter system.
We summed up this principle in the scheme below:
The tRNA amber system preparation
The first step of this preparation was to choose the tranfer RNA and the amino acids to modify in the T7 polymerase gene. We found the article of Grundy and Henkin in 1994 that led us to use the YtRNA. We synthetysed it by an elongation of primers. Now that we know which codon we have to modify within the T7 polymerase sequence. We looked for those codon in the T7 polymerase sequence and realised a quick change mutagenesis, on it to modify two codons. Indeed we decided that only one modified codon would be too leaky, therefore we changed two of them and in the beginning of the sequence for the ribosome to still be tightly attached to the mRNA.
 "
"