Team:Paris Bettencourt/Project
From 2011.igem.org
| Line 2: | Line 2: | ||
<html> | <html> | ||
| - | |||
| - | |||
| - | < | + | <h1>Overview of the project</h1> |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | <p>Mankind is only beginning to grasp the complexity of living organisms. New discoveries often challenge our understanding of life. We believe that synthetic biology can be used as a powerful and reliable tool to help us comprehend and characterize the phenomena we just encountered.</p> | ||
| + | <p>As an iGEM team, we decided to work on one of the most intriguing microbiology discovery of the last decade: the existence of <em>nanotubes communication routes</em> in <i>Bacillus subtilis</i>!</p> | ||
| + | <p>The recent discovery of nanotubes between individual <i>Bacillus subtilis</i> by Dubey and Ben-Yehuda spiked our interest. Through very detailed and advanced microscopy, they showed nanotubes forming between cells and that a wide range of proteins could pass through this communication channel (GFP, calcein, antibiotics, ...). They also showed signs of communication between <i>B.subtilis</i> and <i>E.coli</i>, an entirely different species. This counter-intuitive communication channel could very well have a tremendous impact on evolution, with two different species sharing proteins and even genetic material.</p> | ||
| + | <br> | ||
| + | <center><a href="https://2011.igem.org/File:Nanotube_and_GFP.jpeg"><img src="../wiki/images/e/ed/Nanotube_and_GFP.jpeg" style="width:70%; margin: 15px 15px 0px 15px;"></a></center> | ||
| + | <p><b><center><u>Fig1:</u> <i>B.subtilis</i> exchanging molecules through nanotubes network</center></b></p> | ||
| + | <br> | ||
| + | <br> | ||
| + | <h4>Summary of the article:</h4> | ||
| + | <p>The article published by Dubey and Ben-Yehuda <a href="https://2011.igem.org/Team:Paris_Bettencourt/Project#references">[1]</a> in the Journal <i>Cell</i> is the starting point of our project. In this paper, they show an extraordinary new form of communication between <i>Bacillus subtilis</i> cells and even exchanges with <i>E. coli</i> </p> | ||
| + | <br> | ||
| + | <br> | ||
| + | <div style="margin-left:50px; margin-right:50px; padding: 5px; border:2px solid black;"><b><p>The article in <em>5 bullet points</em> (all of this happens on solid medium only): | ||
<ul> | <ul> | ||
| - | <li>< | + | <li>GFP and calcein, two molecules which can not leave cytoplasm, can be transfered to neighbouring cells in <i>B.subtilis</i>.</li> |
| - | <li> | + | <li>A nanotube network can be observed through electronic microscopy between <i>B.subtilis</i> cells.</li> |
| - | <li> | + | <li>GFP can be observed passing through these nanotubes.</li> |
| - | <li> | + | <li>Antibiotic resistance can be transfered between <i>B.subtilis</i> cells or between <i>B.subtilis</i> and <i>E.coli</i>, both in a hereditary and a non-hereditary way.</li> |
| - | < | + | <li>Nanotubes connecting different species (<i>B.subtilis</i>, <i>E.coli</i> and <i>S.aureus</i>) have been oberved with electronic microscopy.</li> |
| - | < | + | </ul></p></b></div> |
| - | + | <br> | |
| - | + | <br> | |
| - | < | + | <div style="float:left; width: 250px; margin: 15px 15px 0px 15px;"><a href="https://2011.igem.org/Team:Paris_Bettencourt/GFP_microscopy_Dubey_Ben-Yehuda"><img src="https://static.igem.org/mediawiki/2011/e/e0/BY_fluo_gfp_subt.png" style="width:100%;"></a> |
| - | + | <p><b><center><u>Fig2:</u> Visualizing a Molecular Gradient between Neighboring <i>Bacillus subtilis</i> Cells (from the Dubey-Ben-Yehuda article) <a href="https://2011.igem.org/Team:Paris_Bettencourt/Project#references">[1]</a></center></b></p></div> | |
| - | + | ||
| - | < | + | |
| - | + | ||
| - | </ | + | |
| - | + | ||
| - | <a href="https://2011.igem.org/ | + | |
| - | <p>< | + | |
| - | + | ||
| - | + | ||
| - | < | + | |
| - | + | ||
<p> | <p> | ||
| - | < | + | The starting point of this paper was the culture of two different strains of <i>B.subtilis</i>. One produces GFP, a fluorescent protein and the other does not. When grown close together on a solid medium, a <em>transfer of fluorescence</em> from the <i>gfp+</i> towards the <i>gfp-</i> neighbouring cells was observed. Interestingly, this transfer was clearly linked to the distance between two different individuals. |
| - | < | + | </p> |
| - | < | + | <p>To test if this transfer could be reproduced with smaller molecules, the Dubey/Ben-Yehuda team worked with calcein. Calcein is much smaller than GFP (623 Da to compare to 27kDa). Calcein can be used to label cells as it easily enters the cell but does not leave the cytoplasm afterwards. In addition calcein can be hydrolysed by <i>B.subtilis</i>, resulting in a strong fluorescence. Calcein-free cells growing on a solid medium near calcein-labeled cells exhibited the same behaviour as in the GFP experiment above. Non-fluorescent cells exhibited fluorescence and calcein-labeled cells were less fluorescent as time passed. Controls however showed that <i>calcein+</i> cells kept a steady fluorescence and <i>calcein-</i> cells were not fluorescent. |
| - | </ | + | |
</p> | </p> | ||
| - | |||
| - | |||
| - | |||
| - | |||
<br> | <br> | ||
| - | <p> | + | <p> These two experiments suggested that a <em>cell-to-cell close range communication pathway</em> exists in <i>B.subtilis</i>. The Dubey/Ben-Yehuda team investigated this discovery further using electronic microscopy.</p> |
| - | + | <div style="float:right; width: 300px; margin: 15px 15px 0px 15px;"><a href="https://2011.igem.org/Team:Paris_Bettencourt/Electronic_microscopy_Dubey_Ben-Yehuda"><img src="https://static.igem.org/mediawiki/2011/f/f6/BY_electronic.png" style="width:100%;"></a> | |
| - | + | <p><b><center><u>Fig3:</u> Electronic microscopy from the Dubey-Ben-Yehuda article <a href="https://2011.igem.org/Team:Paris_Bettencourt/Project#references">[1]</a></center></b></p></div> | |
| - | + | <p>The pictures support the existence of numerous nanotubes connecting cells. To ensure that these nanotubes could be a significant transfer mechanism, another GFP experiment was tried. Similar to the first one, two antibodies were added. One was an anti-GFP antibody, attaching to the GFP molecules. The other was a secondary antibody attaching to the first one and gold-conjugated. This way, individual GFP molecules could be tagged with the gold-conjugated antibody and followed by electronic microscopy. In this case, they observed <em>GFP molecules moving in the nanotubes</em> from one cell to another.</p> | |
| - | + | <p>The images showed that nanotubes were between <em>30 and 130 nm wide</em> and up to <em>1 µm long</em></p> | |
| - | + | <br> | |
| - | + | <p>The next step was to study <em>antibiotic resistance transfer</em>. Trying to see if non-hereditary (through resistance protein sharing) and hereditary (through plasmids) resistance to antibiotics could be passed through this nanotube network, they manipulated different strains of <i>B.subtilis</i>. We reproduced these experiments and some others related to antibiotic resistance and discussed this matter at length <a href="https://2011.igem.org/Team:Paris_Bettencourt/Atb_exp">here</a>.</p> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | < | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | </ | + | |
| - | + | ||
| - | <p>The | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | <p>The | + | |
| - | + | ||
| - | < | + | |
| - | <p> | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
<br> | <br> | ||
| + | <p>Finally, encouraged by the results of these antibiotics experiments, the Dubey/Ben-Yehuda team took another round of fluorescent and electronic microscopy pictures, this time involving <i>B.subtilis</i>, <i>E.coli</i> and <i>S.aureus</i>. <em>Nanotubes connected those different species</em>, even though some are Gram-positive (<i>B.subtilis</i> and <i>S.aureus</i>) and the other is Gram-negative (<i>E.coli</i>)!</p> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <h2>The Project</h2> | ||
| + | <p>The existence of the nanotube network discovered by Dubey and Ben-Yehuda is still discussed. We wanted to <em>use synthetic biology to provide new evidence</em> supporting the existence of a new cell-to-cell communication in <i>Bacillus Subtilis</i> and between <i>Bacillus Subtilis</i> and <i>E.coli</i>. Thus, we want to <em>characterize this communication</em> as best as we can using carefully crafted genetic designs. We also aim at <em>proposing new applications</em> combining synthetic biology and the nanotubes network.</p> | ||
| + | <p>Each step of our project corresponds to a new level of understanding of the nanotube network inner mechanisms.</p> | ||
| - | |||
| - | <p> | + | <h3>Preliminary experiments</h3> |
| + | <p>Firstly, we aimed to <em>reproduce</em> the observations made in the initial paper. To this end, we reproduced the <a href="https://2011.igem.org/Team:Paris_Bettencourt/Atb_exp">antibiotic</a> and the <a href="https://2011.igem.org/Team:Paris_Bettencourt/GFP_diff">GFP</a> experiments. We also introduced new hypotheses whenever possible and came up with possible explanations for our results.</p> | ||
| + | <p>Since we did not have access to an electronic microscopy facility, we were not able to reproduce the most striking pictures of the article. However, we were determined to obtain quantitative and reliable results with synthetic biology approach.</p> | ||
| - | |||
| - | |||
| - | + | <h3>Characterization</h3> | |
| + | <p>Our second aim was to <em>characterize</em> the nanotubes: what passes through them and what are the typical diffusion times through the network. We tested if RNA, proteins of different sizes and/or metabolites can pass through and with which ease and rate. The idea is to pass different molecules so that we can characterize the speed and the transfer mechanism. For that purpose, we engineered, using synthetic biology approaches <a href="http://en.wikipedia.org/wiki/BioBrick">[2]</a>, different designs built on this logic:</p> | ||
| + | <ul><li>An emitter cell that produces a messenger (RNA, protein etc.) </li> | ||
| + | <li>This messenger passes through the nanotubes and into the receiver cell</li> | ||
| + | <li>The emitter cell has specific promoters that activates an amplification system</li> | ||
| + | <li>This amplification system in turn trigger a detection mechanism we can measure (fluroescence, others)</li></ul> | ||
| + | <center><img src="https://static.igem.org/mediawiki/2011/e/e6/Schema-presentation.png" style="width:800px"></center> | ||
| + | <p><b><center><u>Fig4:</u> Global idea for our designs</center></b></p> | ||
| + | <p>Even though the inter-species (<i>B.subtilis-E.coli</i>) connection seemed more difficult to be reproduced according to the Dubey/Ben-Yehuda paper, we decided to explore it along with the <i>B.subtilis-B.subtilis</i> connection. This was mainly motivated by the overwhelming number of Biobricks available for <i>E.coli</i> when compared to those avalaible for <i>B.subtilis</i>.</p> | ||
| + | <p>An overview of these steps of the project is available <a href="https://2011.igem.org/Team:Paris_Bettencourt/Designs">here</a>.</p> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| + | <h3>Proposing a model for transfer mechanism</h3> | ||
| + | <p>The results from the Dubey/Ben-Yehuda paper suggest that there can be an active process that makes the transfer of the cell constituents from the first one to the second faster than the simple diffusion. This hypothesis is to be taken with caution. A completely active process would involve specific transporters whereas the variety of the molecules transported indicate that there are probably no specificity in the transport.</p> | ||
| + | <p>We chose to <em>investigate an alternative mechanism</em>. Based on tension difference between the lipid membrane of two neighbouring cells, we propose a model that could justify the quick transfer through the nanotubes. We call it "assisted diffusion".</p> | ||
| + | <p>The detailed explanation for this assisted diffusion model is available <a href="https://2011.igem.org/Team:Paris_Bettencourt/Modeling/Assisted_diffusion">here</a>.</p> | ||
| - | <p> | + | <div id="citation_box"> |
| - | + | <p id="references">References</p> | |
| - | < | + | <ol> |
| - | + | <li><i>Intercellular Nanotubes Mediate Bacterial Communication</i>, Dubey and Ben-Yehuda, Cell, available <a href="http://bms.ucsf.edu/sites/ucsf-bms.ixm.ca/files/marjordan_06022011.pdf">here</a></li> | |
| - | < | + | </ol> |
| - | + | </div> | |
<br> | <br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<!-- PAGE FOOTER -- ITEMS FROM COLUMN ! HAVE BEEN MOVED HERE -- RDR --> | <!-- PAGE FOOTER -- ITEMS FROM COLUMN ! HAVE BEEN MOVED HERE -- RDR --> | ||
| Line 224: | Line 135: | ||
</div> | </div> | ||
| - | + | <div id="scroll_right"><a href="https://2011.igem.org/Team:Paris_Bettencourt/Designs"><img src="https://static.igem.org/mediawiki/2011/e/e0/Arrow-right-big.png" style="width:100%;"></a><a href="https://2011.igem.org/Team:Paris_Bettencourt/Designs">To the designs!</a></div> | |
| - | <div id="scroll_right"><a href="https://2011.igem.org/Team:Paris_Bettencourt/ | + | |
</html> | </html> | ||
Revision as of 20:27, 21 September 2011

Overview of the project
Mankind is only beginning to grasp the complexity of living organisms. New discoveries often challenge our understanding of life. We believe that synthetic biology can be used as a powerful and reliable tool to help us comprehend and characterize the phenomena we just encountered.
As an iGEM team, we decided to work on one of the most intriguing microbiology discovery of the last decade: the existence of nanotubes communication routes in Bacillus subtilis!
The recent discovery of nanotubes between individual Bacillus subtilis by Dubey and Ben-Yehuda spiked our interest. Through very detailed and advanced microscopy, they showed nanotubes forming between cells and that a wide range of proteins could pass through this communication channel (GFP, calcein, antibiotics, ...). They also showed signs of communication between B.subtilis and E.coli, an entirely different species. This counter-intuitive communication channel could very well have a tremendous impact on evolution, with two different species sharing proteins and even genetic material.
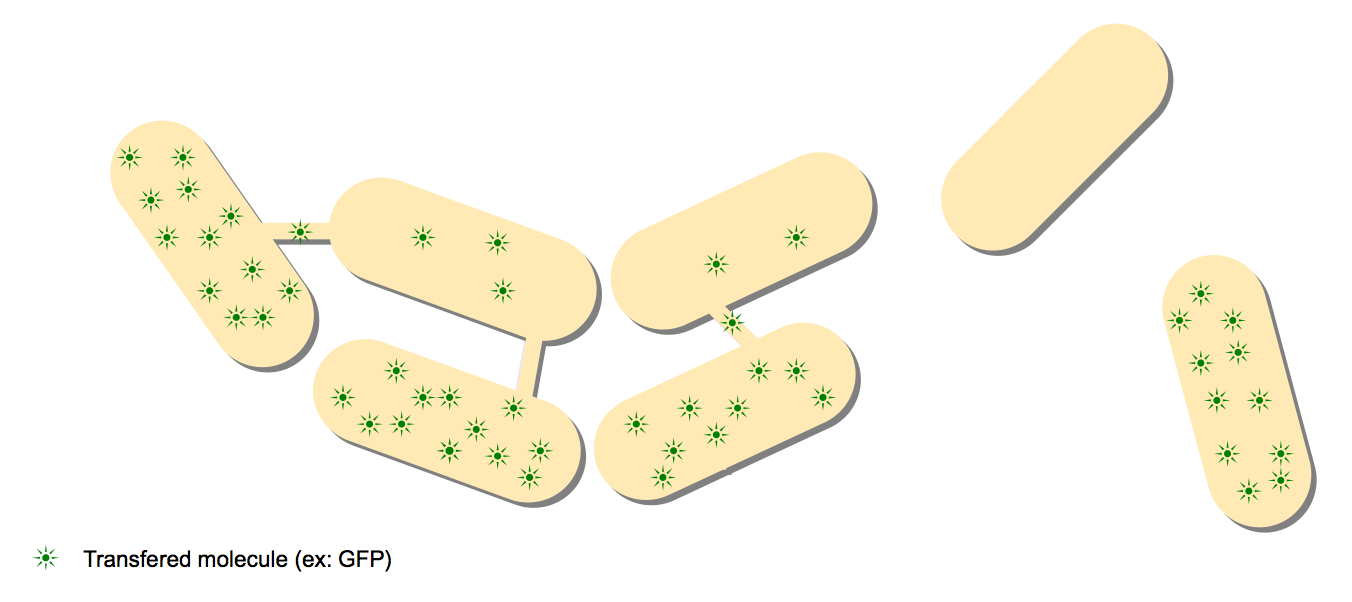
Summary of the article:
The article published by Dubey and Ben-Yehuda [1] in the Journal Cell is the starting point of our project. In this paper, they show an extraordinary new form of communication between Bacillus subtilis cells and even exchanges with E. coli
The article in 5 bullet points (all of this happens on solid medium only):
- GFP and calcein, two molecules which can not leave cytoplasm, can be transfered to neighbouring cells in B.subtilis.
- A nanotube network can be observed through electronic microscopy between B.subtilis cells.
- GFP can be observed passing through these nanotubes.
- Antibiotic resistance can be transfered between B.subtilis cells or between B.subtilis and E.coli, both in a hereditary and a non-hereditary way.
- Nanotubes connecting different species (B.subtilis, E.coli and S.aureus) have been oberved with electronic microscopy.

The starting point of this paper was the culture of two different strains of B.subtilis. One produces GFP, a fluorescent protein and the other does not. When grown close together on a solid medium, a transfer of fluorescence from the gfp+ towards the gfp- neighbouring cells was observed. Interestingly, this transfer was clearly linked to the distance between two different individuals.
To test if this transfer could be reproduced with smaller molecules, the Dubey/Ben-Yehuda team worked with calcein. Calcein is much smaller than GFP (623 Da to compare to 27kDa). Calcein can be used to label cells as it easily enters the cell but does not leave the cytoplasm afterwards. In addition calcein can be hydrolysed by B.subtilis, resulting in a strong fluorescence. Calcein-free cells growing on a solid medium near calcein-labeled cells exhibited the same behaviour as in the GFP experiment above. Non-fluorescent cells exhibited fluorescence and calcein-labeled cells were less fluorescent as time passed. Controls however showed that calcein+ cells kept a steady fluorescence and calcein- cells were not fluorescent.
These two experiments suggested that a cell-to-cell close range communication pathway exists in B.subtilis. The Dubey/Ben-Yehuda team investigated this discovery further using electronic microscopy.
The pictures support the existence of numerous nanotubes connecting cells. To ensure that these nanotubes could be a significant transfer mechanism, another GFP experiment was tried. Similar to the first one, two antibodies were added. One was an anti-GFP antibody, attaching to the GFP molecules. The other was a secondary antibody attaching to the first one and gold-conjugated. This way, individual GFP molecules could be tagged with the gold-conjugated antibody and followed by electronic microscopy. In this case, they observed GFP molecules moving in the nanotubes from one cell to another.
The images showed that nanotubes were between 30 and 130 nm wide and up to 1 µm long
The next step was to study antibiotic resistance transfer. Trying to see if non-hereditary (through resistance protein sharing) and hereditary (through plasmids) resistance to antibiotics could be passed through this nanotube network, they manipulated different strains of B.subtilis. We reproduced these experiments and some others related to antibiotic resistance and discussed this matter at length here.
Finally, encouraged by the results of these antibiotics experiments, the Dubey/Ben-Yehuda team took another round of fluorescent and electronic microscopy pictures, this time involving B.subtilis, E.coli and S.aureus. Nanotubes connected those different species, even though some are Gram-positive (B.subtilis and S.aureus) and the other is Gram-negative (E.coli)!
The Project
The existence of the nanotube network discovered by Dubey and Ben-Yehuda is still discussed. We wanted to use synthetic biology to provide new evidence supporting the existence of a new cell-to-cell communication in Bacillus Subtilis and between Bacillus Subtilis and E.coli. Thus, we want to characterize this communication as best as we can using carefully crafted genetic designs. We also aim at proposing new applications combining synthetic biology and the nanotubes network.
Each step of our project corresponds to a new level of understanding of the nanotube network inner mechanisms.
Preliminary experiments
Firstly, we aimed to reproduce the observations made in the initial paper. To this end, we reproduced the antibiotic and the GFP experiments. We also introduced new hypotheses whenever possible and came up with possible explanations for our results.
Since we did not have access to an electronic microscopy facility, we were not able to reproduce the most striking pictures of the article. However, we were determined to obtain quantitative and reliable results with synthetic biology approach.
Characterization
Our second aim was to characterize the nanotubes: what passes through them and what are the typical diffusion times through the network. We tested if RNA, proteins of different sizes and/or metabolites can pass through and with which ease and rate. The idea is to pass different molecules so that we can characterize the speed and the transfer mechanism. For that purpose, we engineered, using synthetic biology approaches [2], different designs built on this logic:
- An emitter cell that produces a messenger (RNA, protein etc.)
- This messenger passes through the nanotubes and into the receiver cell
- The emitter cell has specific promoters that activates an amplification system
- This amplification system in turn trigger a detection mechanism we can measure (fluroescence, others)

Even though the inter-species (B.subtilis-E.coli) connection seemed more difficult to be reproduced according to the Dubey/Ben-Yehuda paper, we decided to explore it along with the B.subtilis-B.subtilis connection. This was mainly motivated by the overwhelming number of Biobricks available for E.coli when compared to those avalaible for B.subtilis.
An overview of these steps of the project is available here.
Proposing a model for transfer mechanism
The results from the Dubey/Ben-Yehuda paper suggest that there can be an active process that makes the transfer of the cell constituents from the first one to the second faster than the simple diffusion. This hypothesis is to be taken with caution. A completely active process would involve specific transporters whereas the variety of the molecules transported indicate that there are probably no specificity in the transport.
We chose to investigate an alternative mechanism. Based on tension difference between the lipid membrane of two neighbouring cells, we propose a model that could justify the quick transfer through the nanotubes. We call it "assisted diffusion".
The detailed explanation for this assisted diffusion model is available here.
References
- Intercellular Nanotubes Mediate Bacterial Communication, Dubey and Ben-Yehuda, Cell, available here
 "
"


