Team:Paris Bettencourt/Experiments/SinOp
From 2011.igem.org
(Created page with "{{:Team:Paris_Bettencourt/tpl_test}} <html> <h1>SinOp</h1> <p>You can find the details of the experiments on the <a href="https://2011.igem.org/Team:Paris_Liliane_Bettencourt/Not...") |
|||
| Line 1: | Line 1: | ||
{{:Team:Paris_Bettencourt/tpl_test}} | {{:Team:Paris_Bettencourt/tpl_test}} | ||
| - | + | __NOTOC__ | |
| - | + | ==Results== | |
| - | + | === Preparation of slides === | |
| + | Dilution of overnight cultures : YC164 and YC227 (SinOp Design) . <br> | ||
| + | YC227 produces SinI which enhances biofilm formation. If SinI diffuses through the nanotubes we expect to see fluorescence from Peps-gfp construct in the receiver cells. <br> | ||
| + | Two well slides : 1-control (YC164 with IPTG) 2-Mix (both strains with IPTG) | ||
| + | <br> | ||
| + | === Observation === | ||
| + | -37°C Microscopy-<br> | ||
| + | We observed the plate with TRANS and YFP-filter settings on the Old Zeiss. Unfortunately, this microscope gets quickly out of focus so we need to take each picture manually. As expected, YC227 was producing GFP at the begining of the experiment, while YC164 was not. Our observations over 4 hours are the following: | ||
| + | * YC164 grows fast (several divisions over the experiments) | ||
| + | * YC227 does not grow at all and keeps its fluorescence pretty well | ||
| + | * YC164 does not change its state (no obvious biofilm formation) | ||
| + | * After letting the plate overnight we still see some florescence but not as if YC164 changed its fluorescence status. | ||
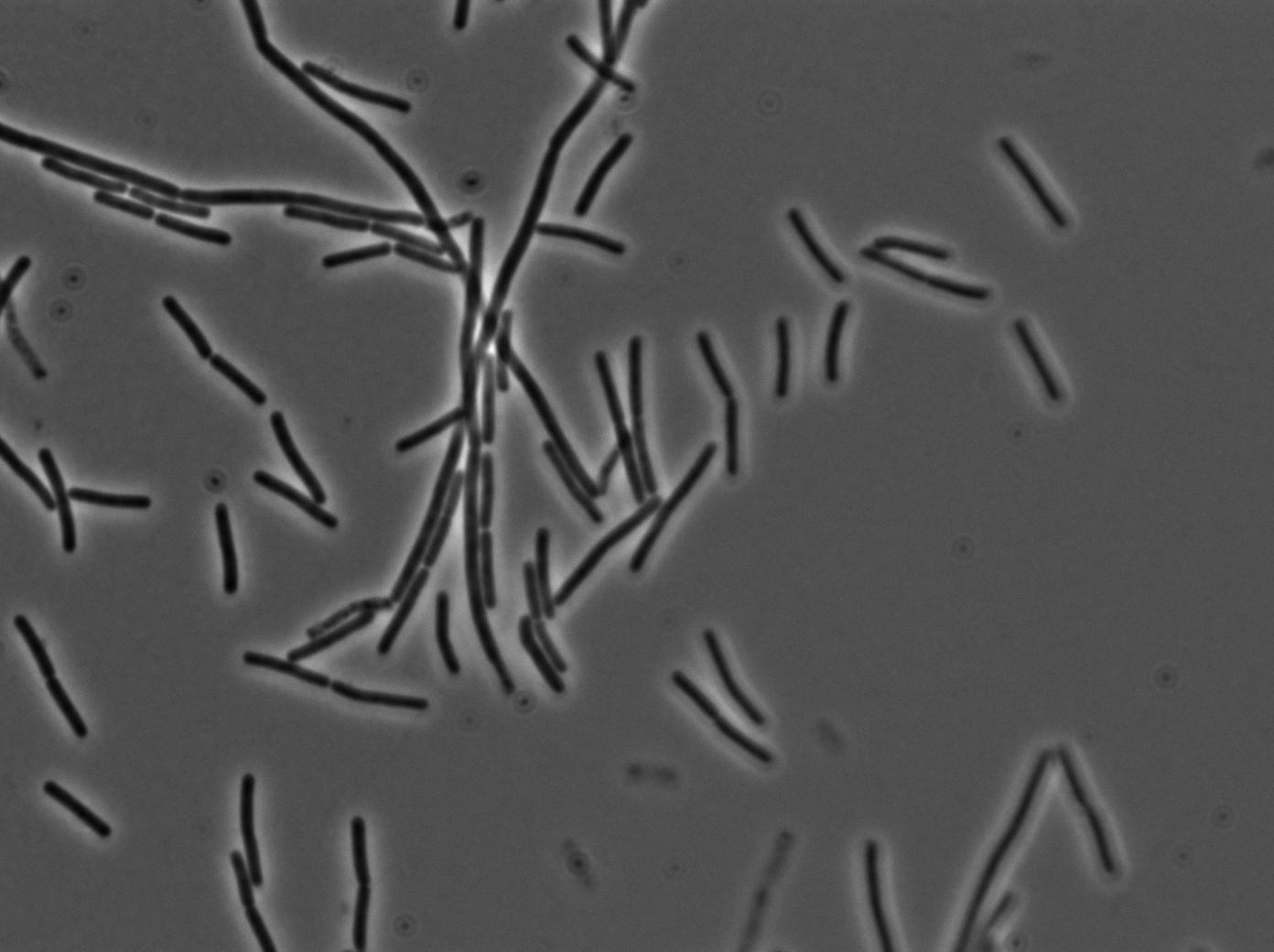
| + | [[File:0905_SinOp_trans1.jpg|500px|thumb|center|TRANS at t=0min]] | ||
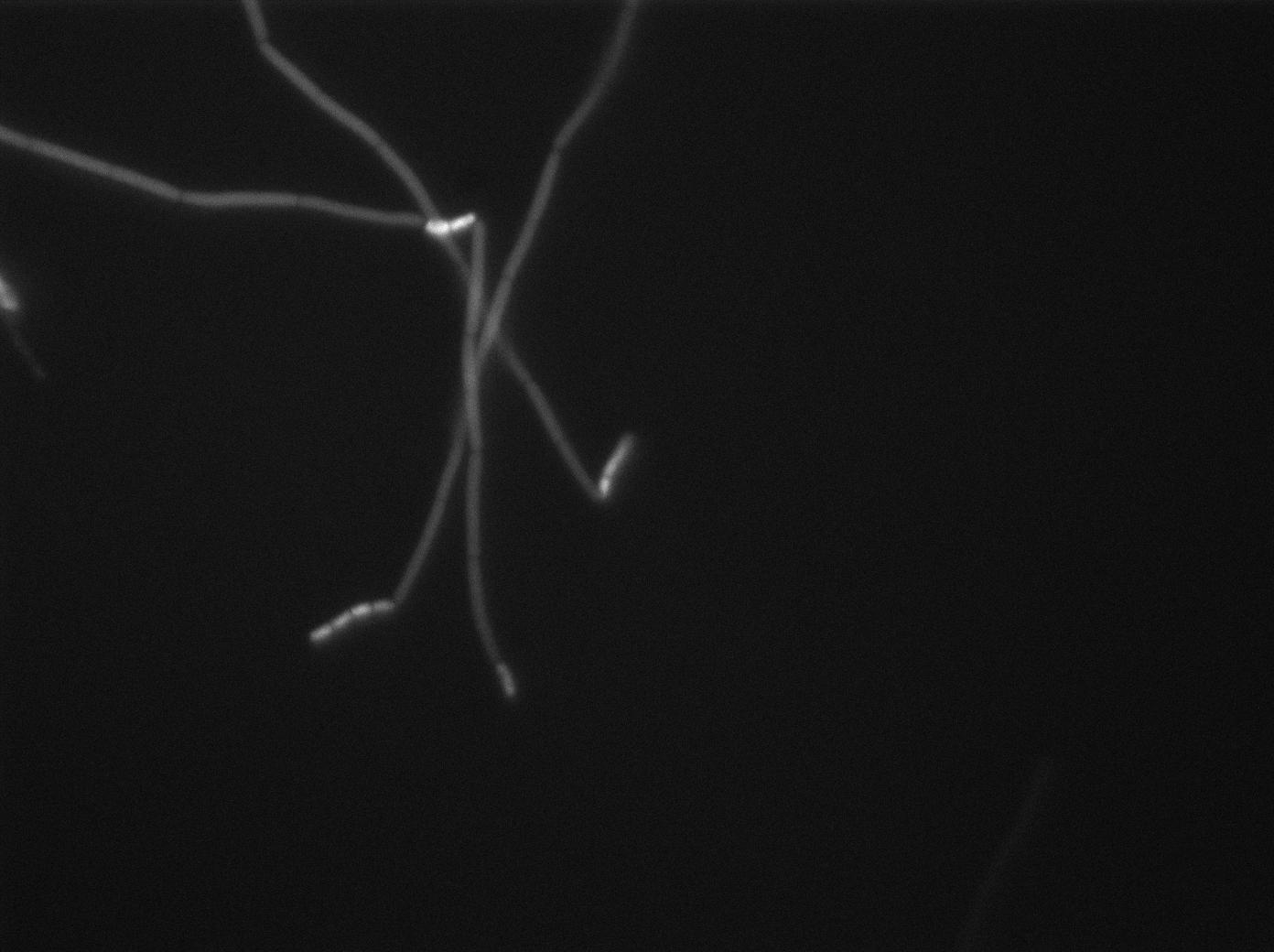
| + | [[File:0905_SinOp_GFP1.jpg|500px|thumb|center|GFP at t=0min]] | ||
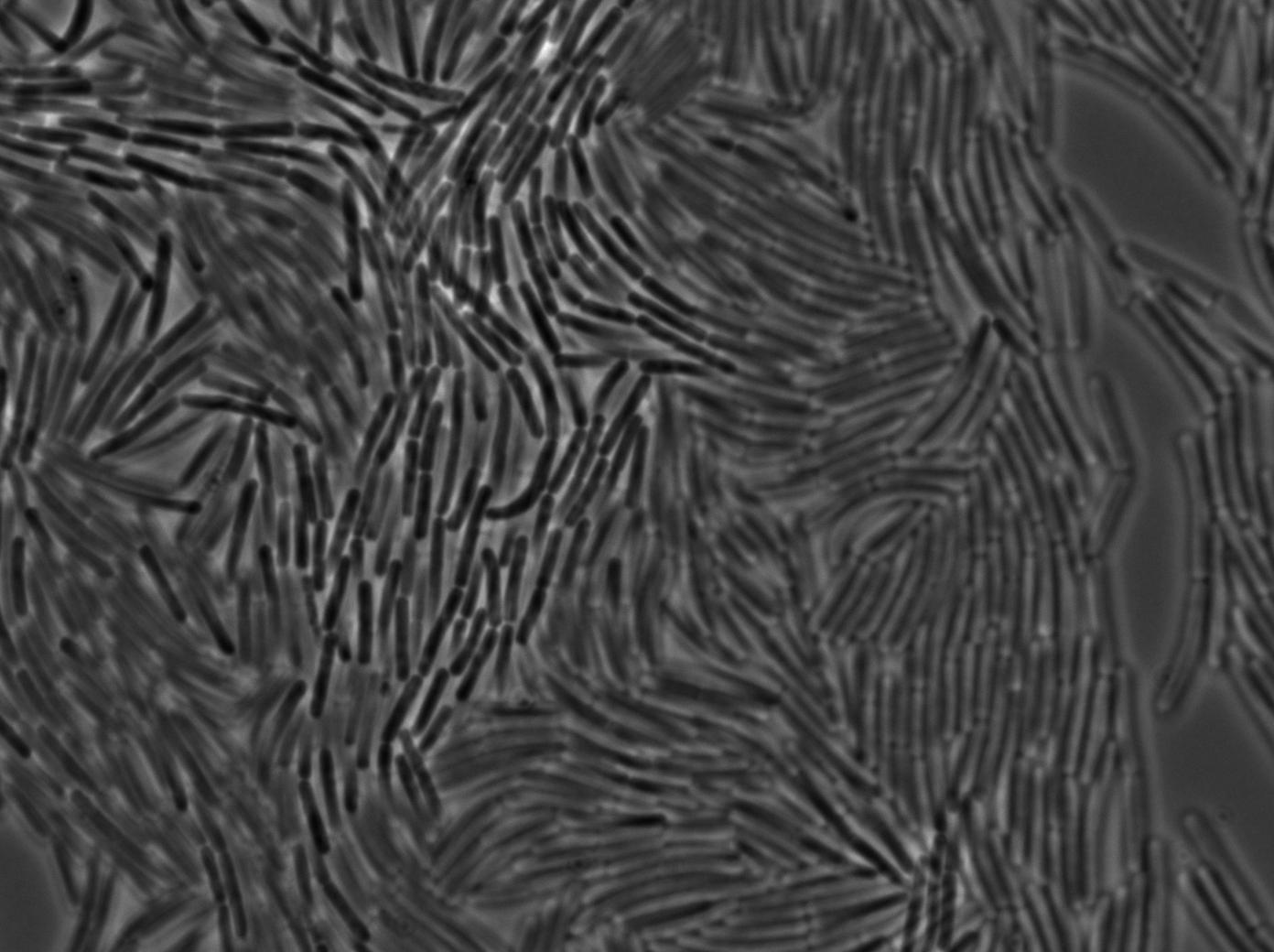
| + | [[File:0905_SinOp_trans14.jpg|500px|thumb|center|TRANS at t=90min]] | ||
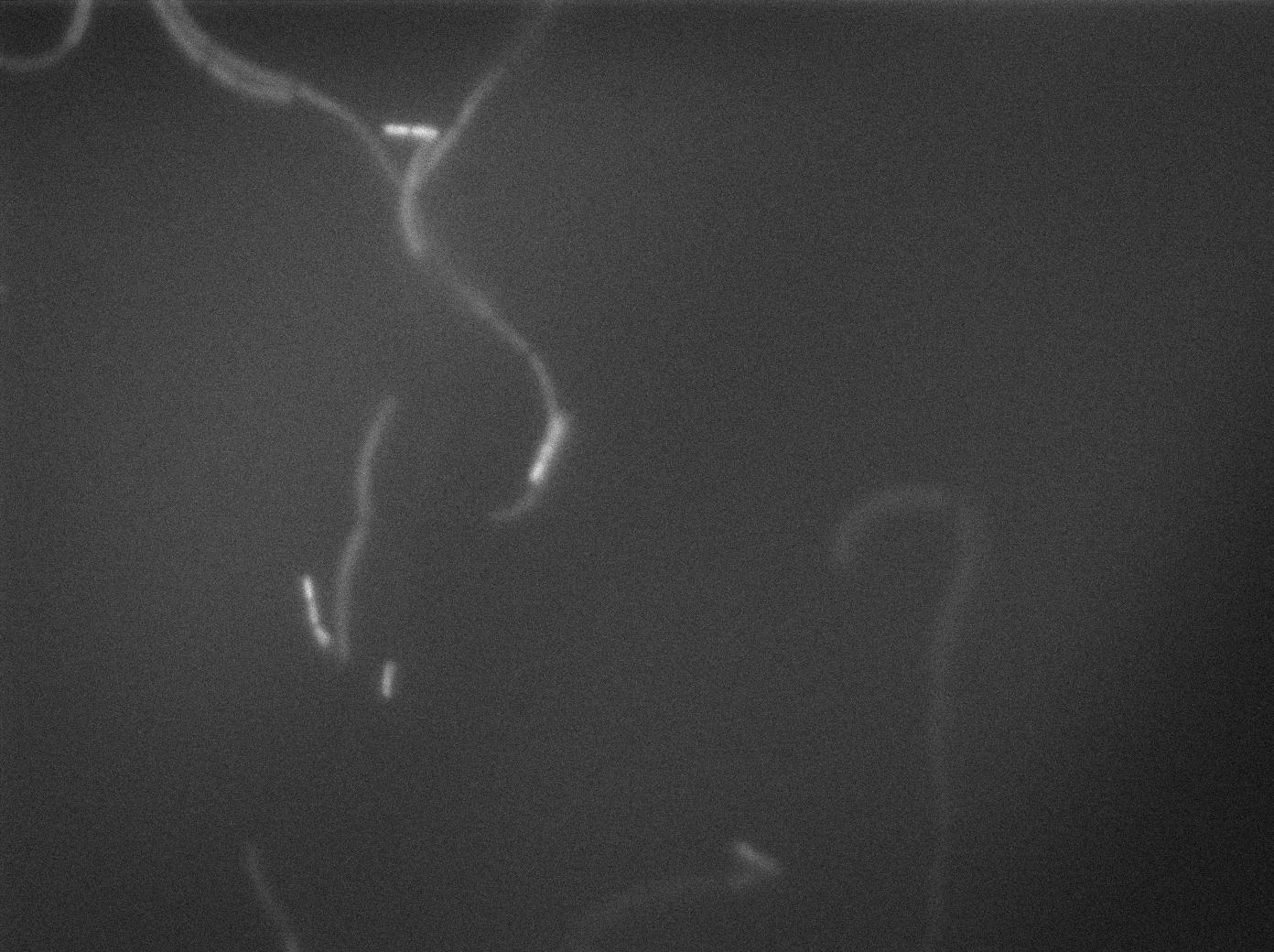
| + | [[File:0905_SinOp_GFP14.jpg|500px|thumb|center|GFP at t=90min]] | ||
| + | |||
| + | === Our conclusions === | ||
| + | We might have poorly chosen the exact phase growth or our timing when plating the strains. Nanotube formation seems to require a more tightly packed colony. We will try this tomorrow. Biofilm formation is usually observed at lower temperatures and it could mean we will never see anything with this design (be reminded that we did not do any cloning for this construction, we only add to reuse strains from another scientific team). | ||
| + | |||
| + | <html> | ||
<!-- PAGE FOOTER -- ITEMS FROM COLUMN ! HAVE BEEN MOVED HERE -- RDR --> | <!-- PAGE FOOTER -- ITEMS FROM COLUMN ! HAVE BEEN MOVED HERE -- RDR --> | ||
| Line 58: | Line 78: | ||
</div> <!-- close footer --> | </div> <!-- close footer --> | ||
</div> <!-- close footer-wrapper --> | </div> <!-- close footer-wrapper --> | ||
| - | </div> | + | |
| - | <div id="scroll_right"><a href="https://2011.igem.org/Team:Paris_Bettencourt/ | + | </div> |
| - | + | <div id="scroll_right"><a href="https://2011.igem.org/Team:Paris_Bettencourt/Designs"><img src="https://static.igem.org/mediawiki/2011/e/e0/Arrow-right-big.png" style="width:100%;"></a><a href="https://2011.igem.org/Team:Paris_Bettencourt/Designs">To the designs!</a></div> | |
</html> | </html> | ||
Revision as of 01:49, 22 September 2011

Results
Preparation of slides
Dilution of overnight cultures : YC164 and YC227 (SinOp Design) .
YC227 produces SinI which enhances biofilm formation. If SinI diffuses through the nanotubes we expect to see fluorescence from Peps-gfp construct in the receiver cells.
Two well slides : 1-control (YC164 with IPTG) 2-Mix (both strains with IPTG)
Observation
-37°C Microscopy-
We observed the plate with TRANS and YFP-filter settings on the Old Zeiss. Unfortunately, this microscope gets quickly out of focus so we need to take each picture manually. As expected, YC227 was producing GFP at the begining of the experiment, while YC164 was not. Our observations over 4 hours are the following:
- YC164 grows fast (several divisions over the experiments)
- YC227 does not grow at all and keeps its fluorescence pretty well
- YC164 does not change its state (no obvious biofilm formation)
- After letting the plate overnight we still see some florescence but not as if YC164 changed its fluorescence status.
Our conclusions
We might have poorly chosen the exact phase growth or our timing when plating the strains. Nanotube formation seems to require a more tightly packed colony. We will try this tomorrow. Biofilm formation is usually observed at lower temperatures and it could mean we will never see anything with this design (be reminded that we did not do any cloning for this construction, we only add to reuse strains from another scientific team).
 "
"