Team:Paris Bettencourt/Experiments/Methodologies/Tecan
From 2011.igem.org

Kinetics
What we call the Tecan® is the Infinite 200 PRO multimode reader from Tecan®.
This machine can read fluorescence and absorbance in a 96 well plate, within cycles that are programmed to be repeated, and the kinetics can last for hours. It allows to look at evolution of fluorescence during all the growth of a bacterial colony.
In our case as the receiver and the emitter of each of our system have specific fluorescence, we tuned the Tecan® to measure fluorescence every 5 or 10 minutes for 3 to 12 hours depending on what we were studying. To characterize a part, we usually do an overnight experiment. To test wether nanotubes are forming or not, 3 to 4 hours are enough since we need B.subtilis to be in exponential phase.
The sensitivity of the Tecan® allows precise quantification of the fluorescence emitted by our various designs.
Nanotube experiment
What we want to measure is the appearance of fluorescence in receiver system (switch off -> on). But to switch the receiver system on, we needed to make sure we could reproduce the conditions of nanotubes formation in the 200 microliters wells of the 96 plate. We therefore first tested the growth of GFP+ Bacillus subtilis in three different conditions to see if they could grow normally :
- cells in rich media (LB), concentrated at the bottom of the well by centrifugation
- cells in rich media (LB), with on the bottom of the well a thin layer of LB agar, concentrated by centrifugation again
- cells in rich media without centrifugation
Our negative controls were LB without bacteria, and LB agar and LB without bacteria. We fine tuned the Tecan sensitivity for each experiments. At the end of experiments we resuspend the cells and measure the optical density. We are hence able to compare the cells growth in each conditions.
According to the curve the LB agar wasn't important for the cell's growth.
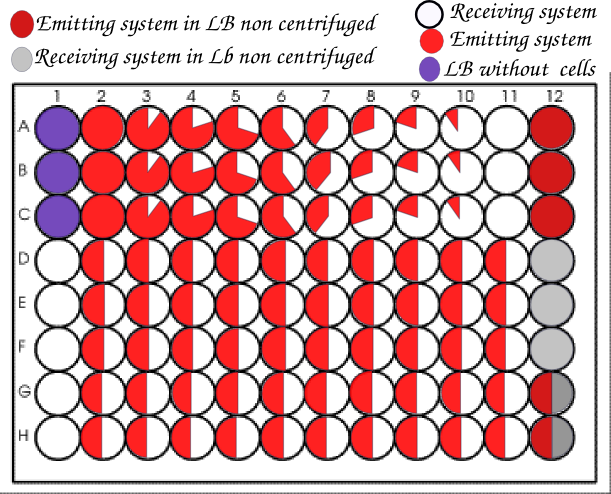
We then realized in parallel of the microscopy team all the experiments they were doing with the same systems, but as we had 96 wells to do so we tried different ratios of strains. For each ratio we did at least one triplicate.
We usually organized our plate as below.
We realized so much wells with a ratio 1:1 to get the closest possible to the conditions of the article.
To reproduce the nanotube experiment, we let grow the strains to reach the exponential phase and then we dilute them in the wells for them to continue the exponential growth, for the 3 to 4 hours that lasts the measure.
Characterisation experiment
To characterise our parts we let the strains containing them growing to an OD of 0.2 that is before subtilis or coli exponential phase to be able to well see it, an then put them in the plate. That characterisation allowed us to know if our systems were working. Indeed we managed to put a fluorescent marker below each system therefore we could measure the evolution of fluorescence during growth, and more specifically during exponential phase(phase of the nanotubes).
 "
"