Team:Paris Bettencourt/Experiments/Methodologies/Tecan
From 2011.igem.org
(→Nanotube experiment) |
m |
||
| Line 28: | Line 28: | ||
We realized so much wells with a ratio 1:1 to get the closest possible to the conditions of the article. | We realized so much wells with a ratio 1:1 to get the closest possible to the conditions of the article. | ||
| - | <br><br><br> | + | To reproduce the nanotube experiment, we let grow the strains to reach the exponential phase and then we dilute them in the wells for them to continue the exponential growth, for the 3 to 4 hours that last the measure. |
| + | |||
| + | <br> | ||
| + | ==Characterisation experiment== | ||
| + | |||
| + | |||
| + | To characterise our parts we let the strains containing them growing to an OD of 0.2 that is before <i>subtilis</i> exponential phase to be able to well see it, an then put them in the plate. | ||
| + | |||
| + | If our system requires an induction to switch on, we use strains that are induced before being put in the wells and non induced ones that we would induce in the plate itself. | ||
| + | <br><br> | ||
| + | |||
| + | |||
<html> | <html> | ||
<!-- PAGE FOOTER -- ITEMS FROM COLUMN ! HAVE BEEN MOVED HERE -- RDR --> | <!-- PAGE FOOTER -- ITEMS FROM COLUMN ! HAVE BEEN MOVED HERE -- RDR --> | ||
Revision as of 00:48, 29 October 2011

What we call the Tecan is the Infinite 200 PRO multimode reader from Tecan®.
This machine can read fluorecence and absorbance in a 96 well plate, within cycles that are programmed to be repeated, and the kinetics can last for hours. It allows to look at evolution of fluorescence during all the growth of a bacterial colony.
In our case as the receiver and the emitter of each of our system have specific fluorescence, we tuned the Tecan to measure fluorescence every 5 or 10 minutes for 3 to 12 hours depending on what we were studying. Indeed, to characterize a part, we do the characterization overnight. If we wanted to do a nanotube experiment, it had to last the exponential phase of B. subtilis - 3 to 4 hours were enough.
The advantage of the Tecan over the other techniques is the quantification of the fluorescence observed.
Nanotube experiment
What we want to measure with the Tecan is the appearance of fluorescence in receiver system (switch off -> on). But to switch on the receiver system we need the conditions of the nanotube experiment to be reproduced in the 200 microliter well of the 96 plate. We first tested the growth of GFP+ Bacillus subtilis in three different conditions to see if they could grow normally, the conditions were :
- cells in rich media (LB) centrifuged
- cells in rich media, with on the bottom of the well a thin layer of LB agar, centrifuged
- cells in rich media without centrifugation
Our controls were LB without bacteria, and LB agar and LB without bacteria. We tuned the Tecan for it to measure at the good level even when there was agar in the bottom and ran the experiment for 12 hours. At the end of the 12 hours we resuspended the cells and measured the optical density to be able to compare if the cells grew approximatively the same according to the conditions.
According to the curve the LB agar wasn't important for the cell's growth.
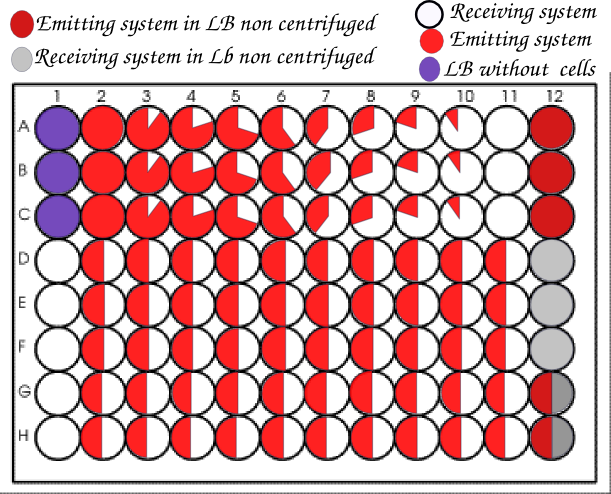
We then realized in parallel of the microscopy team all the experiments they were doing with the same systems, but as we had 96 wells to do so we tried different ratios of strains. For each ratio we did at least one triplicate.
We usually organized our plate as below.
We realized so much wells with a ratio 1:1 to get the closest possible to the conditions of the article.
To reproduce the nanotube experiment, we let grow the strains to reach the exponential phase and then we dilute them in the wells for them to continue the exponential growth, for the 3 to 4 hours that last the measure.
Characterisation experiment
To characterise our parts we let the strains containing them growing to an OD of 0.2 that is before subtilis exponential phase to be able to well see it, an then put them in the plate.
If our system requires an induction to switch on, we use strains that are induced before being put in the wells and non induced ones that we would induce in the plate itself.
 "
"