Team:Harvard/Technology
From 2011.igem.org
Overview | MAGE | Chip-Based Synthesis | Lambda Red | Protocols
Zinc Finger Binding Site Finder
Check out our Zinc Finger Binding Site Finder Tool here! This tool was used to search the human genome for the six target DNA sequences that we used to design our custom zinc finger arrays.
MAGE
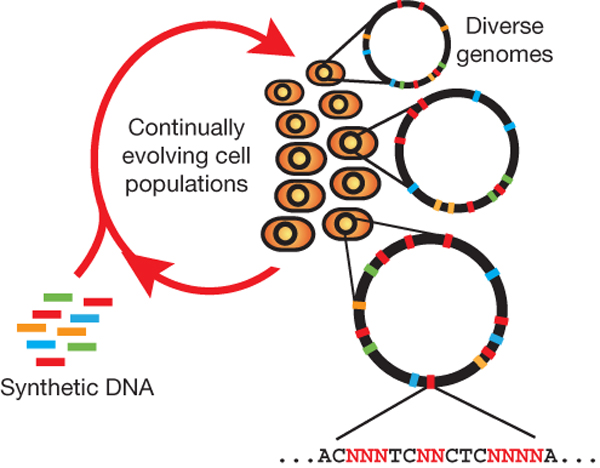
Summary (Adapted from Wang et al)2,3
The breadth of genomic diversity found among organisms in nature allows populations to adapt to diverse environments. However, genomic diversity is difficult to generate in the laboratory and new phenotypes do not easily arise on practical timescales. Although in vitro and directed evolution methods have created genetic variants with usefully altered phenotypes, these methods are limited to laborious and serial manipulation of single genes and are not used for parallel and continuous directed evolution of gene networks or genomes.
Multiplex automated genome engineering (MAGE) is a new method for large-scale programming and evolution of cells. MAGE simultaneously targets many locations on the chromosome, thus producing combinatorial genomic diversity. Because the process is cyclical and scalable, MAGE facilitates rapid and continuous generation of a diverse set of genetic changes (mismatches, insertions, deletions). This multiplex approach embraces engineering in the context of evolution by expediting the design and evolution of organisms with new and improved properties.
MAGE provides a highly efficient, inexpensive and automated solution to simultaneously modify many genomic locations (for example, genes, regulatory regions) across different length scales, from the nucleotide to the genome level.
Lambda Red- Mediated Recombineering
Useful Links
- Gene Knockouts and Exchanges by Linear Transformation: http://rothlab.ucdavis.edu/protocols/Lin.Transform.html
- Open Wet Ware Protocol: http://openwetware.org/wiki/Recombineering/Lambda_red-mediated_gene_replacement
References
1. Sriram Kosuri, Nikolai Eroshenko, Emily M LeProust, Michael Super, Jeffrey Way, Jin Billy Li, George M Church. (2010). Scalable gene synthesis by selective amplification of DNA pools from high-fidelity microchips. Nature Biotechnology, 28(12):1295-9. [1]
2. Harris H. Wang, Farren J. Isaacs, Peter A. Carr, Zachary Z. Sun, George Xu, Craig R. Forest, George M. Church. Programming cells by multiplex genome engineering and accelerated evolution. (2009). Nature, 460(7257):894-8. [2]
3. Isaacs FJ, Carr PA, Wang HH, Lajoie MJ, Sterling B, Kraal L, Tolonen AC, Gianoulis TA, Goodman DB, Reppas NB, Emig CJ, Bang D, Hwang SJ, Jewett MC, Jacobson JM, Church GM. (2011). Precise manipulation of chromosomes in vivo enables genome-wide codon replacement. Science, 333(6040):348-53. [3]
4. Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA 3rd, Smith HO. (2009). Enzymatic assembly of DNA molecules up to several hundred kilobases. Nature Methods,6(5):343-5. Epub 2009 Apr 12.
[4]
5. Gibson D. (2009). One-step enzymatic assembly of DNA molecules up to several hundred kilobases in size. Nature Protocols,Published online 16 April 2009, doi:10.1038/nprot.2009.77.
[5]
 "
"








