Team:Harvard/Results/Biobricks
From 2011.igem.org
(→Zinc Finger-Omega Subunit Fusion Protein (BBa_K615001)) |
|||
| Line 1: | Line 1: | ||
{{:Team:Harvard/Template:PracticeBar2}} | {{:Team:Harvard/Template:PracticeBar2}} | ||
| - | + | {{:Team:Harvard/Template:ResultsGrayBar}} | |
| - | + | ||
| - | + | ||
<div class="whitebox"> | <div class="whitebox"> | ||
Revision as of 00:44, 26 September 2011
Overview | Biobricks | Source Code | Acknowledgments | Accomplishments
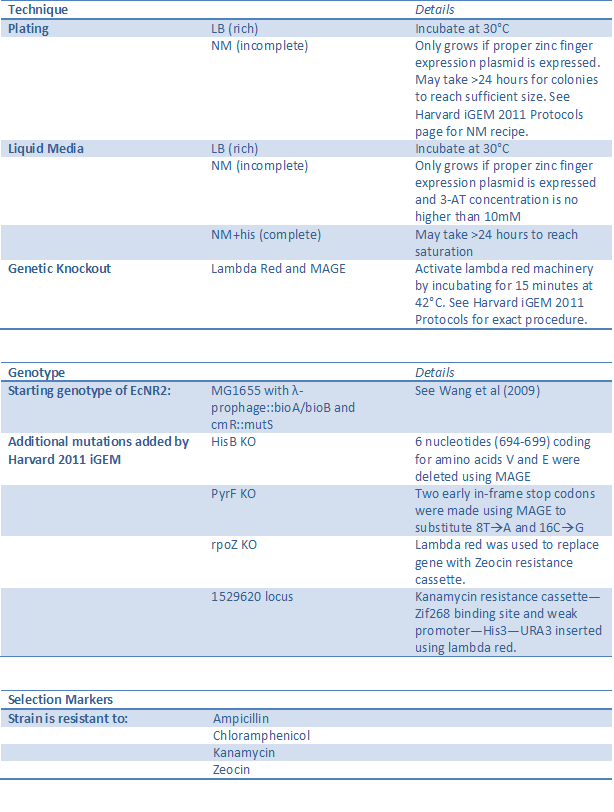
One-hybrid selection system strain (BBa_K61500)
[http://partsregistry.org/Part:BBa_K615000 See our chassis page] in the Registry of Standard Biological Parts.
The His3-URA3 one-hybrid selection system has been developed by Meng et al (2005)to test zinc finger binding by tying the binding event to cell survival. However, Meng used a plasmid-based system where both the zinc fingers and the binding site were on vectors within the cell, while we took advantage of recent advances in technology to make this one-hybrid system genome based. This prevents one or both components from being lost during cell division, ensures that each cell has exactly one copy of the zinc finger binding site-His3-URA3 construct, and presents the genome as the next frontier for standardized part construction. (For a full description of the one-hybrid system, see our Selection Strain Engineering page.)
Using lambda red and MAGE, we knocked out HisB and PyrF and replaced with them with the yeast analogs His3 and URA3 under the control of a zinc finger binding site (see our MAGE and our Lambda Red results pages). The omega subunit of RNA polymerase was also knocked out and instead fused to the first zinc finger of the array to be tested (see below). This allows zinc finger binding to control expression of His3 and URA3, and thus in incomplete media the cell will only survive if the proper zinc finger is present.
Our strain was carefully characterized and shown to have the proper phenotype and be sensitive enough to recognize a valid zinc finger when diluted up to one in one million. The selectivity can also be adjusted by changing the concentration of 3-AT, a competitive inhibitor of His3 (see One Hybrid Selection Results for details). See below for a summary of the changes made to the strain and how to successfully use it.
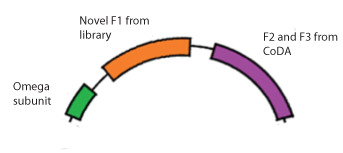
Zinc Finger-Omega Subunit Fusion Protein (BBa_K615001)
[http://partsregistry.org/Part:BBa_K615001 See the Biobrick page] in the Registry of Standard Biological Parts.
To test our library of 55,000 zinc fingers, we designed expression plasmids that contain the second and third zinc fingers (F2 and F3) in the array that are known from CoDA, the novel finger from the library, and the omega subunit. When transformed into the selection strain, the ability of the zinc finger to bind will determine whether RNA polymerase is recruited and thus whether His3 and URA3 are expressed. (See our Chip Library page for construction details.)
This specific part is designed to bind to the sequence 5'-GTG GGA TGG-3', where the GTG-GGA codons have known zinc finger binders, but the GGA-TGG (F2-F1) combination was unknown. Finding a zinc finger that binds to TGG in the context of GGA can also translate into many three-finger arrays, since any known F3-GGA pair can be combined with F2 GGA-F1 TGG.
References:
Xiangdong Meng, Michael H Brodsky, Scot A Wolfe. A bacterial one-hybrid system for determining the DNA-binding specificity of transcription factors. (2005) Nature Biotechnology, 23(8): 988-994.
Jeffry D Sander, Elizabeth J Dahlborg, Mathew J Goodwin et al. Selection-free zinc-finger-nuclease engineering by contextdependent assembly (CoDA). (2011) Nature Methods 8(1): 53-55.
Harris H. Wang, Farren J. Isaacs, Peter A. Carr, Zachary Z. Sun, George Xu, Craig R. Forest, George M. Church. Programming cells by multiplex genome engineering and accelerated evolution. (2009). Nature, 460(7257):894-8.
 "
"