Team:Harvard/Project
From 2011.igem.org
| Line 7: | Line 7: | ||
<div class="whitebox"> | <div class="whitebox"> | ||
=Our Project= | =Our Project= | ||
| - | + | Zinc fingers are specialized proteins that bind to DNA. Due to their ability to target highly specific DNA sequences, zinc fingers offer great potential for gene therapy and personalized medicine: recently, they were shown to be effective in conferring HIV resistance and treating hemophilia in mice. In the past, however, designing new zinc fingers - a necessity for individualized gene therapy - has been prohibitively expensive and time consuming. | |
| - | + | ||
| - | + | For our project, we created and tested thousands of zinc fingers at a cost feasible for most labs. To do so, we harnessed two novel synthetic biology technologies: chip-based synthesis, which allows for thousands (even millions) of DNA strands to be synthesized concurrently, and multiplex automated genome engineering (MAGE), which makes possible direct edits of the genome of organisms, rather than using small, cumbersome plasmids. | |
| - | + | To do this, our project has three main steps: | |
| - | + | ==1. Design== | |
| - | + | '''Use a bioinformatics approach to predict 55,000 zinc finger sequences.''' | |
| - | + | ||
As the structure and binding interactions of zinc fingers are not yet understood, our project also utilizes bioinformatics and computational analysis of the limited existing data to make “educated guesses” to generate zinc fingers that could bind to DNA for genes that cause colorblindness, some types of cancer, and high cholesterol. | As the structure and binding interactions of zinc fingers are not yet understood, our project also utilizes bioinformatics and computational analysis of the limited existing data to make “educated guesses” to generate zinc fingers that could bind to DNA for genes that cause colorblindness, some types of cancer, and high cholesterol. | ||
| + | |||
| + | targeted against six DNA sequences for three diseases | ||
| + | |||
| + | ==2. Synthesize== | ||
| + | '''Use chip-based DNA synthesis to make 55,000 sequences simultaneously, then insert the oligos into E.coli.''' | ||
| + | |||
| + | ===3. Test== | ||
| + | '''Use a metabolic selection system to test which zinc finger sequences successfully bind DNA.''' | ||
| + | |||
| + | |||
Thus, our zinc fingers and their clinical applications are a new technology, and while we hope that our new zinc fingers work, the more important goal is maximizing efficiency and decreasing cost while utilizing new technology: we anticipate that future iGEM teams will find great use for chip-based synthesis and MAGE. | Thus, our zinc fingers and their clinical applications are a new technology, and while we hope that our new zinc fingers work, the more important goal is maximizing efficiency and decreasing cost while utilizing new technology: we anticipate that future iGEM teams will find great use for chip-based synthesis and MAGE. | ||
Revision as of 23:56, 18 October 2011
Overview | Design | Synthesize | Test | Zinc Finger Background | Protocols
Contents |
Our Project
Zinc fingers are specialized proteins that bind to DNA. Due to their ability to target highly specific DNA sequences, zinc fingers offer great potential for gene therapy and personalized medicine: recently, they were shown to be effective in conferring HIV resistance and treating hemophilia in mice. In the past, however, designing new zinc fingers - a necessity for individualized gene therapy - has been prohibitively expensive and time consuming.
For our project, we created and tested thousands of zinc fingers at a cost feasible for most labs. To do so, we harnessed two novel synthetic biology technologies: chip-based synthesis, which allows for thousands (even millions) of DNA strands to be synthesized concurrently, and multiplex automated genome engineering (MAGE), which makes possible direct edits of the genome of organisms, rather than using small, cumbersome plasmids.
To do this, our project has three main steps:
1. Design
Use a bioinformatics approach to predict 55,000 zinc finger sequences.
As the structure and binding interactions of zinc fingers are not yet understood, our project also utilizes bioinformatics and computational analysis of the limited existing data to make “educated guesses” to generate zinc fingers that could bind to DNA for genes that cause colorblindness, some types of cancer, and high cholesterol.
targeted against six DNA sequences for three diseases
2. Synthesize
Use chip-based DNA synthesis to make 55,000 sequences simultaneously, then insert the oligos into E.coli.
=3. Test
Use a metabolic selection system to test which zinc finger sequences successfully bind DNA.
Thus, our zinc fingers and their clinical applications are a new technology, and while we hope that our new zinc fingers work, the more important goal is maximizing efficiency and decreasing cost while utilizing new technology: we anticipate that future iGEM teams will find great use for chip-based synthesis and MAGE.
See here for our abstract and detailed project description.
Technological Applications
The novel methods we employed in our project have the potential to revolutionize synthetic biology practices, and the way that future iGEM competitions are conducted. To learn more about the technological applications of our project, please see our Technology page.
Zinc Finger Background
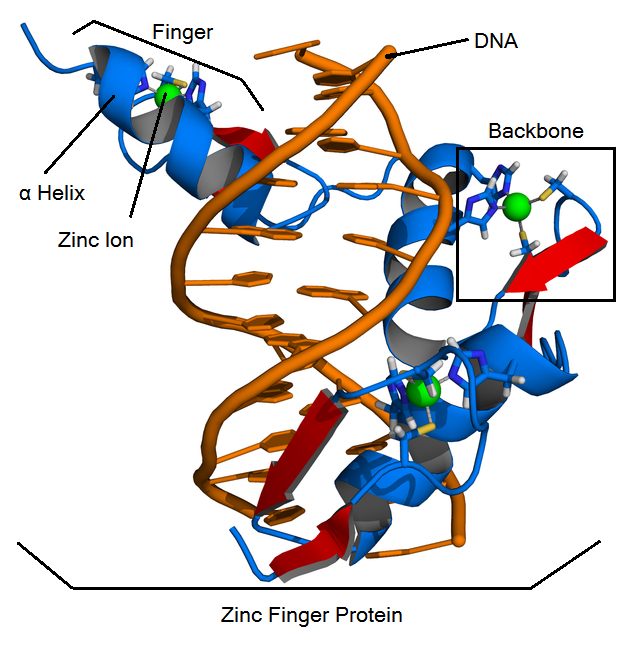
What are Zinc Finger Proteins (ZFPs)?
Function
ZFPs are found commonly in nature as a class of special transcription factors that bind to DNA, thus regulating gene expression. Zinc finger function was first studied using zinc finger protein Zif268.
Structure
ZFPs consist of smaller subunits called "fingers" which each contain a zinc finger binding helix that binds to unique DNA sequences. These fingers are linear and linked together by the "zinc finger backbone", a series of approximately 21 amino acids.
- Cis2His2 ZFPs have three main structural components:
- Zinc finger binding helix
- Linker region
- Zinc ion that is coordinated by two cysteine residues and two histidine residues.
Helpful Zinc Finger Links
[http://en.wikipedia.org/wiki/Zinc_finger Zinc Fingers on Wikipedia]
- A more detailed introduction to zinc fingers.
[http://compbio.cs.princeton.edu/zf/ Predicting DNA Recognition by C2H2 Zinc Finger Proteins]
- A program useful for predicting how well a given amino acid sequence will bind to a given DNA sequence
[http://www.zincfingers.org/default2.htm The Zinc Finger Consortium]
- Information & helpful resources for zinc fingers
[http://www.jounglab.org/ Joung Lab]
- Information about Dr. Joung's extensive work with zinc fingers
Clinical Applications of Zinc Fingers
Colorblindness (Red Opsin)
- Goal: Produce functional red opsin photoreceptor proteins in the eye
- Method: Insertion of functional red opsin gene (OPN1LW [http://genome.ucsc.edu/cgi-bin/hgGene?hgg_geneuc004fjz.3&hgg_protP04000&hgg_chromchrX&hgg_start153409724&hgg_end153424505&hgg_typeknownGene&dbhg19&hgsid206379197 1]) upstream of normal locus in patient lacking the gene
Inherited High Cholesterol (Familial Hypercholesterolemia)
- Goal: Produce functional LDLR protein to remove LDL cholesterol from the blood
- Method: Insertion of functional LDLR gene upstream of nonfunctional allele
Cancer (Myc Oncogene)
- Goal: Knock out the oncogenic protein product and stop cancerous proliferation
- Method: Targeted disruption (deletion) in mutated oncogene
 "
"