Team:ETH Zurich/Biology/Detector
From 2011.igem.org
(→How we used it) |
(→How we used it) |
||
| Line 111: | Line 111: | ||
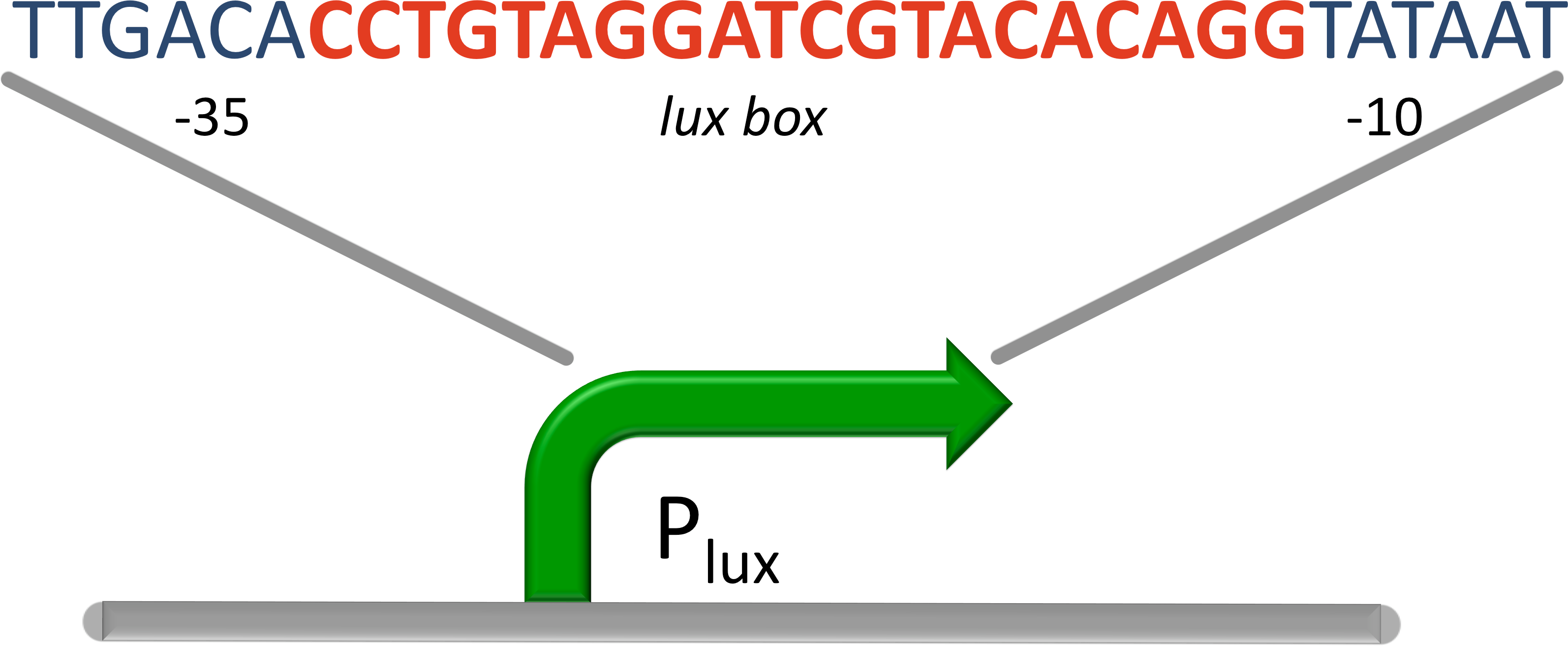
Figure 9: Promoter for luxR''', the operon (red) designed between the -10 and the -35 region (blue) of the promoter.]] | Figure 9: Promoter for luxR''', the operon (red) designed between the -10 and the -35 region (blue) of the promoter.]] | ||
| - | For our system we used a promoter which is repressed by luxR! (BBa_R0061) It was designed by placing the lux box between and partially overlapping the consensus -35 and -10 site of an artificial lacZ promoter. In the presence of AHL, LuxR binds to the lux box and inhibits binding of the RNA Polymerase. [[#Ref11|[11]]] | + | For our system we used a promoter which is repressed by luxR! ([http://partsregistry.org/Part:BBa_R0061 BBa_R0061]) It was designed by placing the lux box between and partially overlapping the consensus -35 and -10 site of an artificial lacZ promoter. In the presence of AHL, LuxR binds to the lux box and inhibits binding of the RNA Polymerase. [[#Ref11|[11]]] |
{{:Team:ETH Zurich/Templates/SectionEnd}} | {{:Team:ETH Zurich/Templates/SectionEnd}} | ||
{{:Team:ETH Zurich/Templates/SectionStart}} | {{:Team:ETH Zurich/Templates/SectionStart}} | ||
Revision as of 18:09, 27 October 2011

Contents |
Network Elements
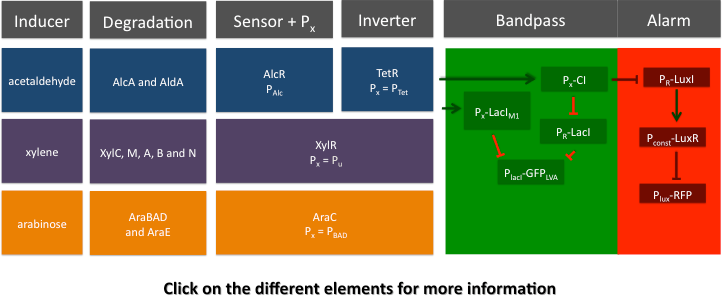
SmoColi consist of several different network elements: the major sub-networks are a sensor, a band-pass filter and a quorum sensing mechanism.
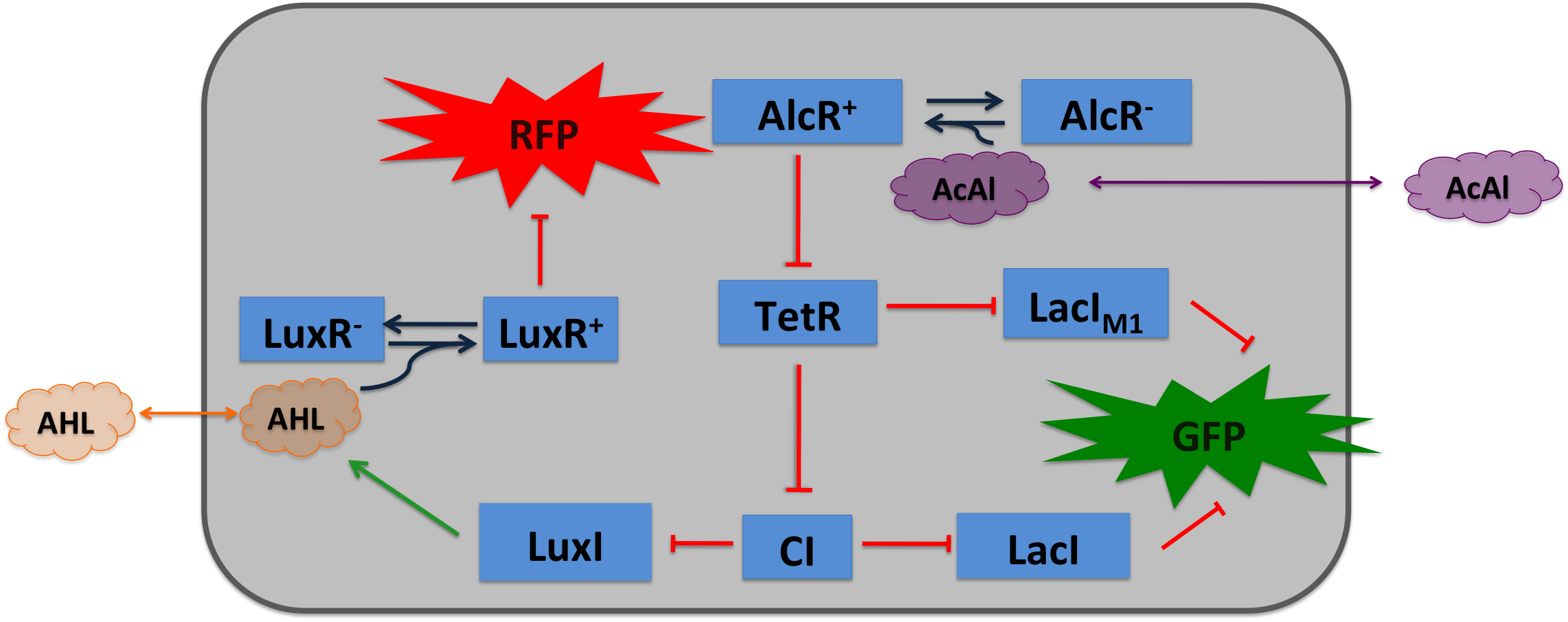
Cigarette smoke contains a lot of different toxic and carcinogenic components. In biology many sensors for such components exist, mostly to induce their degradation. One is the acetaldehyde system in aspergillus nidulans: the activator AlcR binds to its operator site if acetaldehyde is present [1]. Other ones are the xylene sensing system in Pseudomonas putida [2] and the one for styrene in Pseudomonas sp. [3], both also working as activators. However our system can be induced with any Sensor, as a proof of principle we induced it also with arabinose. We combined a smoke-sensitive band-pass filter with GFP output with a quorum-sensing diffusive mechanism that alarms the user of the system to high smoke levels by expressing RFP.
Acetaldehyde Sensor
Where it comes from...
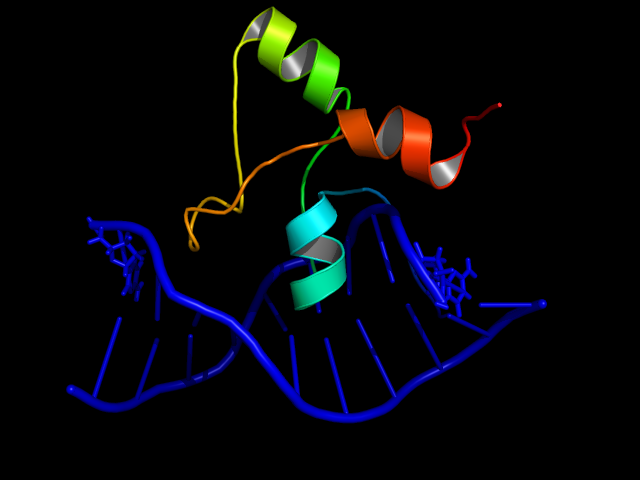
In Aspergillus nidulans the alcR gene encodes a regulatory protein which induces the expression of the ethanol utilization (alc) pathway if the co-inducer acetaldehyde is present. Ethanol is converted to acetaldehyde by the alcohol dehydrogenase, acetaldehyde is afterwards metabolized to acetate by the aldehyde dehydrogenase. Both genes alcA and aldA are regulated by AlcR. AlcR also regulates its own expression by autoactivation [1].
The AlcR DNA binding domain contains a zinc bi-nuclear cluster, which can bind either to symmetric and asymmetric sites with same affinity. In contrast to other members of the Zn2Cys6, the DNA binding domain is a 16 residue long sequence between the third and fourth cysteine. AlcR binds as a monomer, but 2 molecules can bind to inverted repeats in a noncooperative manner. For high-affinity binding additional DNA sequences upstream of the zinc cluster were identified [4].
How we used it
Because fungal activators normally do not work in E. coli, we modified AlcR in a way that it acts as a repressor. The mechanism of how AlcR activates transcription is not completely known, which makes the process of redesigning it very challenging. A second challenge is the different codon usage in Apergillus nidulans and E. coli. The codon adaptation index (CAI) of Aspergillus nidulans AlcR is 0.7 in E. coli, especially at the beginning of the gene a few very rare codons were included. To get a fully E. coli-optimized gene, we ordered the gene codon-optimized. To get a partly codon-optimized version, we exchanged the rare codons at the beginning of the Aspergillus nidulans protein with PCR.
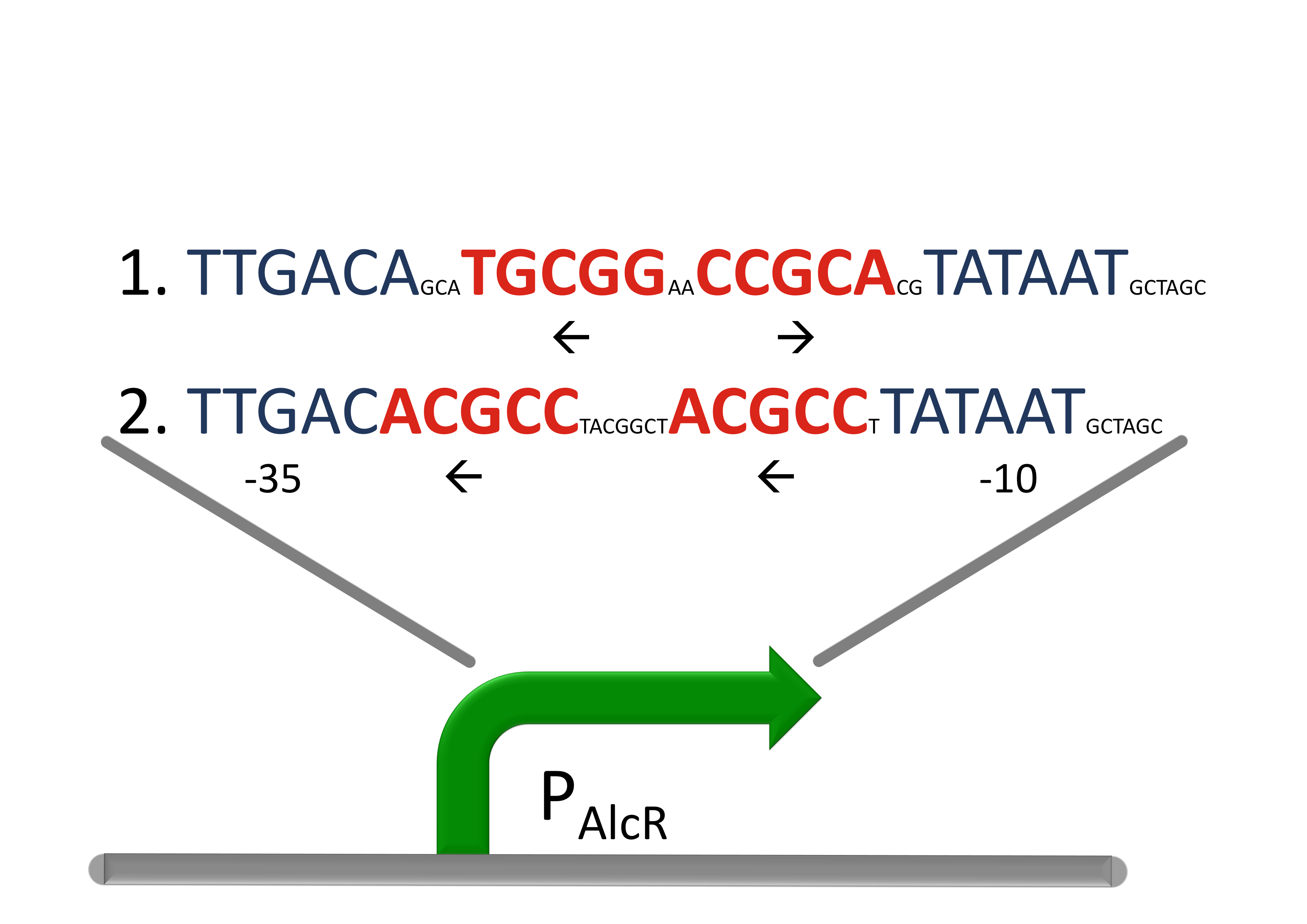
For the redesigned promoter PAlcR, the operator site of AlcR from Aspergillus nidulans was placed between the -10 and -35 region of the bacterial promoter. We used two different versions as DNA targets, one with direct-repeat targets and one with inverted [1]. The idea is that in the presence of acetaldehyde, AlcR will bind to the operon and block the binding of the RNA polymerase. This only works if the mechanism of AlcR binding does not involve conformational changes or anything similar. To make sure that an excess of AlcR is present with respect to PAlcR, they were expressed on different plasmids with different copy numbers. The AlcR promoter is cloned in front of the TetR repressor to introduce the bandpass.
Acetaldehyde is reduced by the native E. coli enzyme aldehyde dehydrogenase, generating a concentration gradient in our tube. At the position with the required concentration of acetaldehyde for the bandpass-filter, GFP is expressed, at all other positions it is repressed, resulting in a single GFP band. By tuning the input of acetaldehyde in the tube, the GFP band moves. If the acetaldehyde concentration reaches a certain very high level, the whole tube expresses RFP.
Xylene Sensor
Where it comes from...
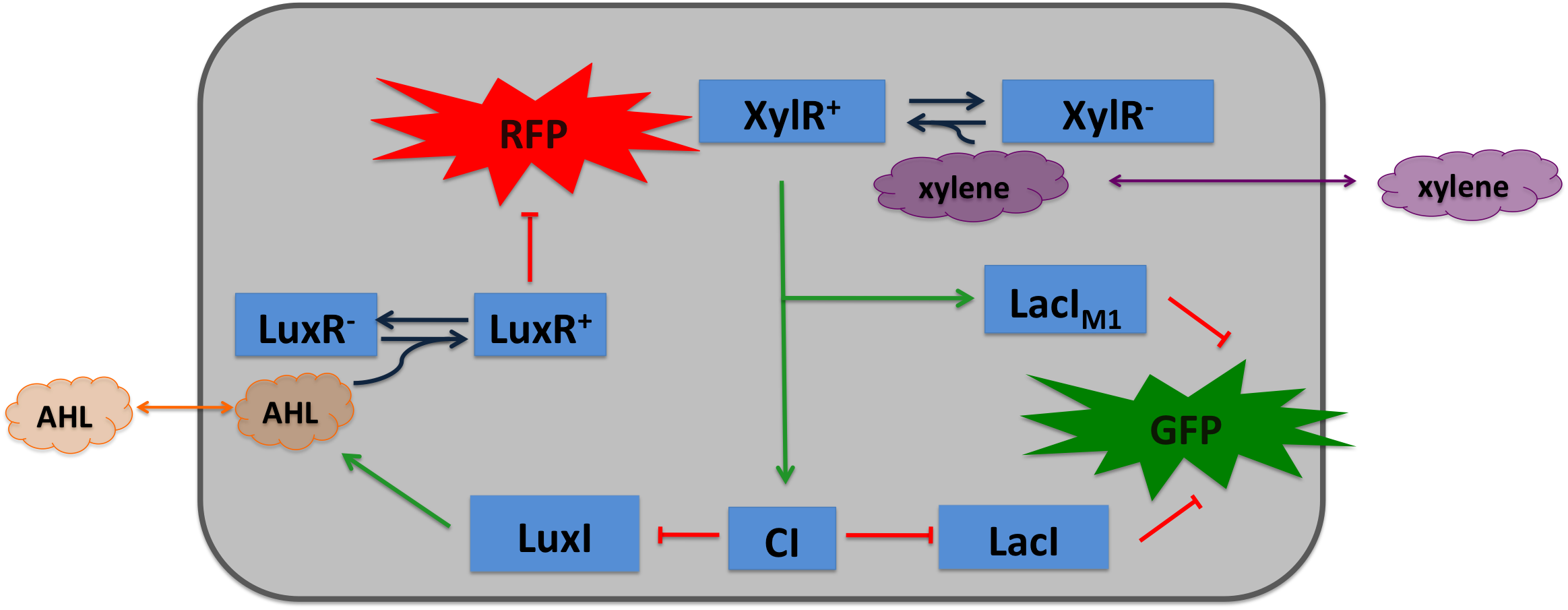
The xylR gene in Pseudomonas putida encodes a 566 aa long regulatory protein that activates the degradation of toluene and xylene. In P. putida the complete upper Tol pathway is under the control of the toluene-responsive promoter PU, in the presence of xylene XylR binds to PU and activates it [5]. Then the five genes xylC-xylM-xylA-xylB-xylN are expressed, where xylC encodes for the benzaldehyde dehydrogenase, xylM and xylA for subunits of the xylene oxygenase and xylB for benzyl alcohol dehydrogenase [6]. XlyN is not required for the degradation of xylene, but it is part of the whole transcription unit. The DNA binding domain of XylR binds to a 40 bp long upstream activating sequence (UAS) located 150 bp upstream from the transcription start site of the σ54 PU promoter. DNA looping between the UAS and the -12/-24 motifs of the σ54-RNAP facilitates the activation of the holoenzyme by XylR [7].
How we used it
Xylene is not degraded naturally by E. coli. To generate a concentration gradient of xylene we engineered its degradation in SmoColi by including the upper Tol pathway. XylR is a prokaryotic enhancer binding protein which works in E. coli as well as in Pseudomonas putida. The LacIM1 and the CI regulators were under the control of the PU promoter, enhancing their expression in the presence of xylene.
Arabinose Sensor
Where it comes from...
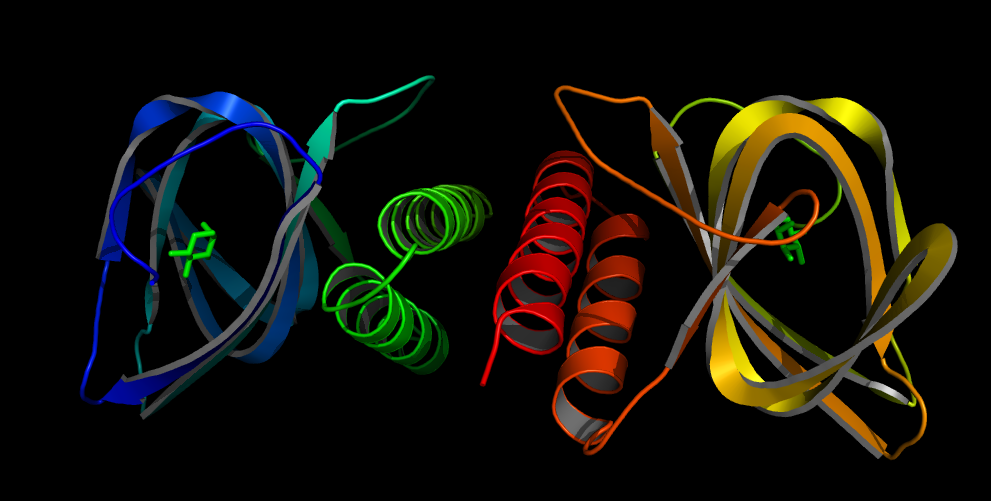
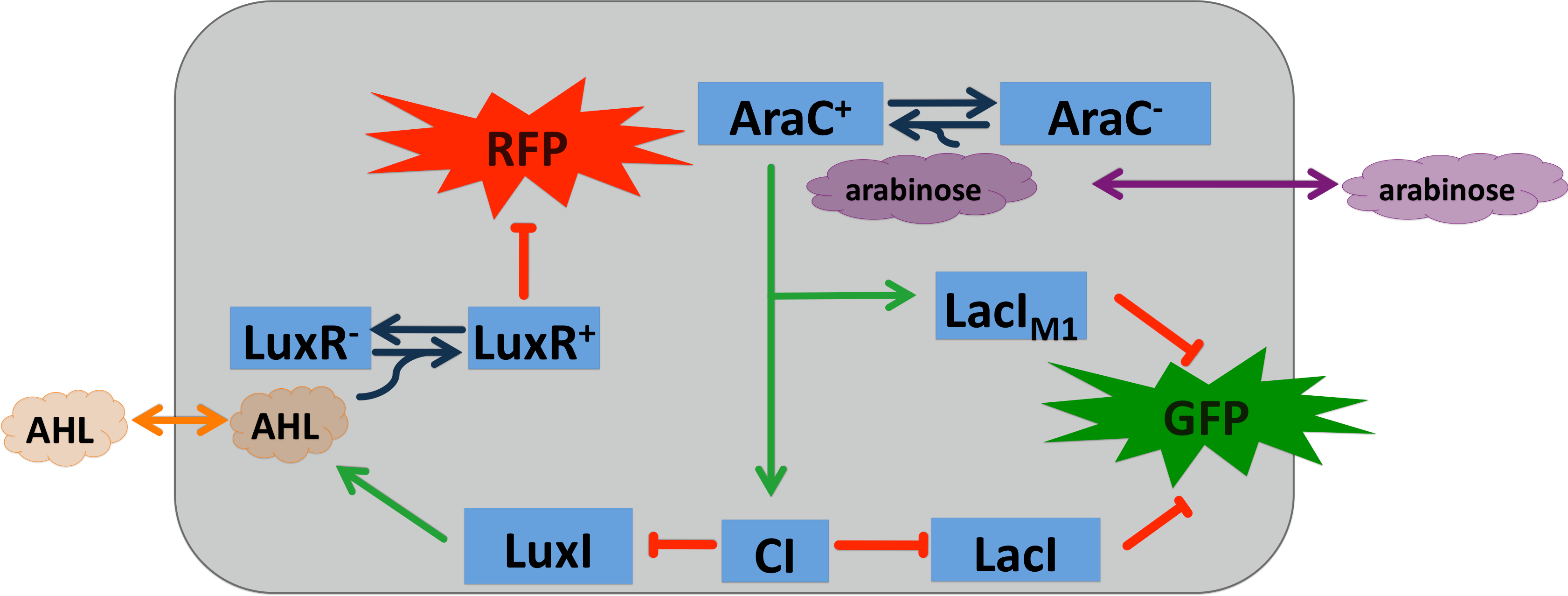
The AraC regulatory protein is a activator of the gens for the araBAD, the araE and the araFGH operon in E.coli. araB, araA and araD encode for the metabolic enzymes which degrade L-Arabinose to D-xylulose-5-phosphate. In absence of arabinose AraC does bind to two DNA site separated over 200 bp from each other (I1 and O2 of the PBAD promoter) [8]. By dimer formation of the two DNA bound AraC molecules the DNA is looped and transcription blocked. Binding of arabinose leads to binding of arabinose to I1 and I2, I2 bound AraC directly interacts with the RNA polymerase and activates it. araF, araG and araH and araE encode for proteins needed for uptake of arabinose. If some arabinose enters the cell, the uptake of arabinose is activated leading and more and more arabinose entering the cell. This positive feedback loop leads to a all or nothing response of arabinose in the cells.
How we used it
To show that our system can be activated by different signals, we introduced a arabinose sensor. Due to the fact that AraC s well established in contract AlcR and XlyR, the arabinose system can be seen as proof of principle for the bandpass and the alarm system for our smoke sensors. The LacIM1 and the CI regulators were under the control of the PBAD promoter, enhancing their expression in the presence of arabinose and the absence of glucose. The all or nothing response of the arabinose system makes the natural system unusable for our bandpass, because we need a intermediate input signal. In order that we can still use the arabinose system we use the BW27783 E.coli stain [9]. This stain has a knock out of the araFGH (transporter) and the PBAD-araE is replaced by PCP8-araE which is a consecutive promoter. Without the positive feedback loop we get a nice dose response for the system, with a intermediate input concentrations for the bandpass filter. By inactivating of the kanamycin casette we created the BW27783 from the BW27749, which was provided to us by Khlebnikov et al. (Done according to the method of: [10])
Bandpass
Where it comes from...
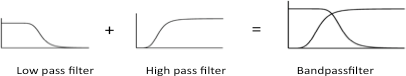
In 2005 Ron Weiss et al. constructed the first band-pass filter device in synthetic biology. This was done by using the combination of a high-pass and a low-pass filter. An input signal is transmitted by cell to cell communication while the signal processing is done using an intracellular feed-forward loop. Filters are created by using different transcriptional regulator proteins with different repression efficiencies and binding affinities for corresponding promoters.
How we used it
We used his pioneering network design as a basis in the planning process for our band-pass filter implementation [11]. Instead of cell-cell communication via AHL for the input signal, we included our different smoke detectors which detect small volatile molecules like acetaldeyhde, xylene or arabinose.
Inverter
Where it comes from...
An inverter is a part which converts a positive signal into a negative one, or vise versa. For SmoColi we used TetR as an inverter. TetR binds and blocks the PTet promoter.
How we used it
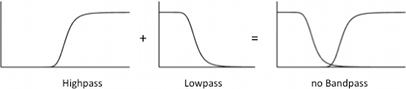
With a negative input to the bandpass filter, the band would disappear,because you only switch the low and high pass filter (see Figure 8). If the negative signal is inverted we get a again a nice bandpassfilter. In our circuits the Inverter is only needed for AlcR, for the activators AraC and XylR the input signal is correct
Quorum Sensing of the Alarm system
Where it comes from...
The luminescence operon in Vibrio fischeri is regulated by the transcriptional activator (LuxR). The lux box is a 20 bp inverted repeat sequence at a position centered 42.5 bases upstream of the transcriptional start. LuxR binds to the lux box in presents of AHL and enhance its expression. LuxR is needed to activate the σ70 RNA Polymerase. The Vibrio fischeri lux operon consists of seven genes, the first one is luxI. The luxI gene encodes for the enzyme for AHL synthesis. The autoinducer AHL is secreted by the cells to coordinate gene expression based on the local density of the bacterial population. Furthermore transcription of luciferase in the lux operon is induced, leading to bioluminescence.
How we used it
For our system we used a promoter which is repressed by luxR! (BBa_R0061) It was designed by placing the lux box between and partially overlapping the consensus -35 and -10 site of an artificial lacZ promoter. In the presence of AHL, LuxR binds to the lux box and inhibits binding of the RNA Polymerase. [11]
References
Pymol:
 "
"