Team:EPF-Lausanne/Our Project/T7 promoter variants/lysis
From 2011.igem.org
(→Experimental Setup) |
(→Results) |
||
| Line 26: | Line 26: | ||
=== Results === | === Results === | ||
| - | [[Image:lysis_dynamics.png|500px]] | + | [[Image:lysis_dynamics.png|500px|center]] |
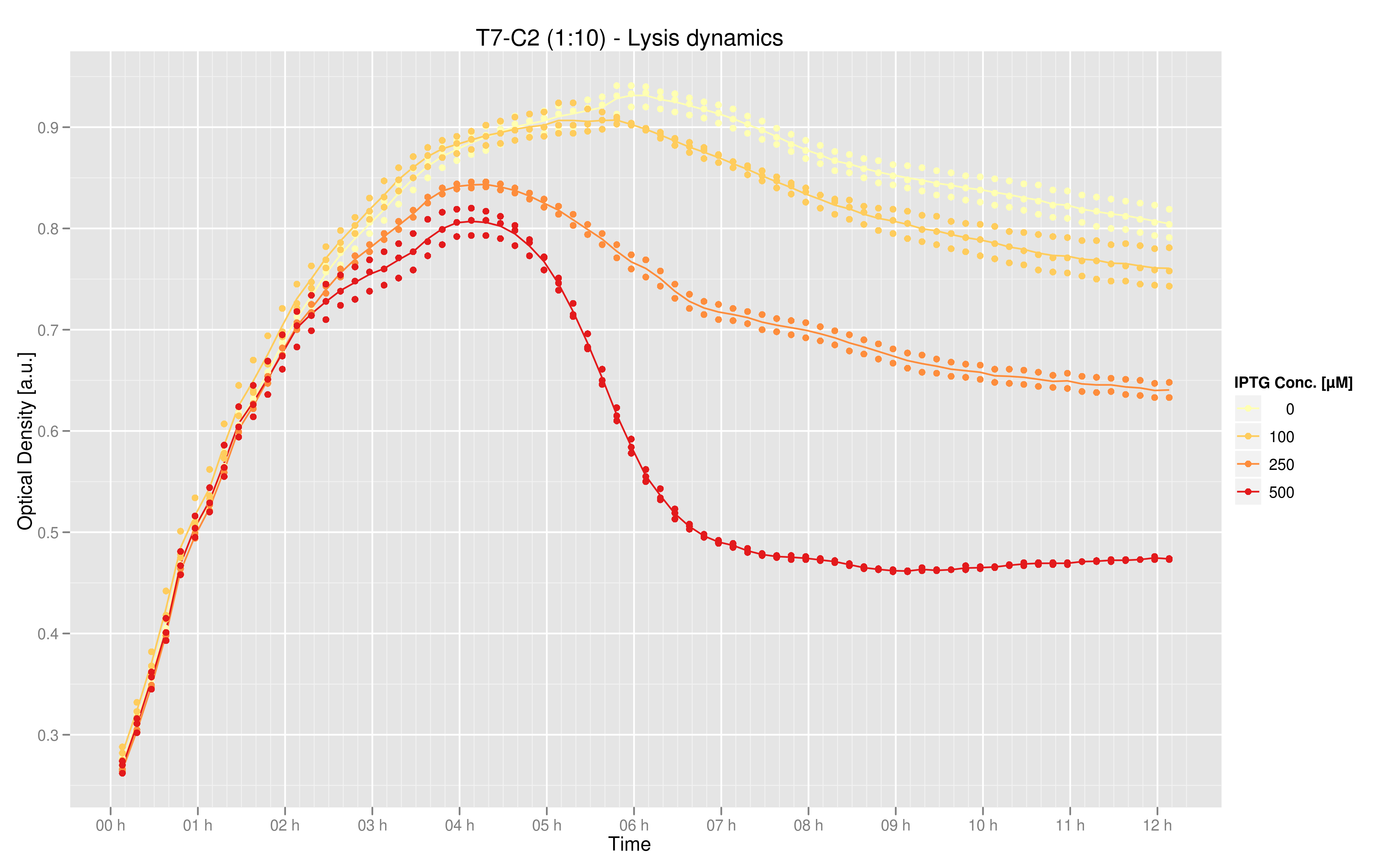
Data was produced in triplicate via the 96 well plate. This graphic shows the mean over the three data points for the measurements taken every 10 minutes. The values on the y-axis are relative fluorescence units (RFU) normalized by optical density (OD), while the x-axis represents time. The curves indicate that maximal lysis is produced with 500 uM IPTG, and that lesser degrees of lysis can be obtained with smaller doses of IPTG. | Data was produced in triplicate via the 96 well plate. This graphic shows the mean over the three data points for the measurements taken every 10 minutes. The values on the y-axis are relative fluorescence units (RFU) normalized by optical density (OD), while the x-axis represents time. The curves indicate that maximal lysis is produced with 500 uM IPTG, and that lesser degrees of lysis can be obtained with smaller doses of IPTG. | ||
| - | [[Image:dose_response.png|500px]] | + | [[Image:dose_response.png|500px|center]] |
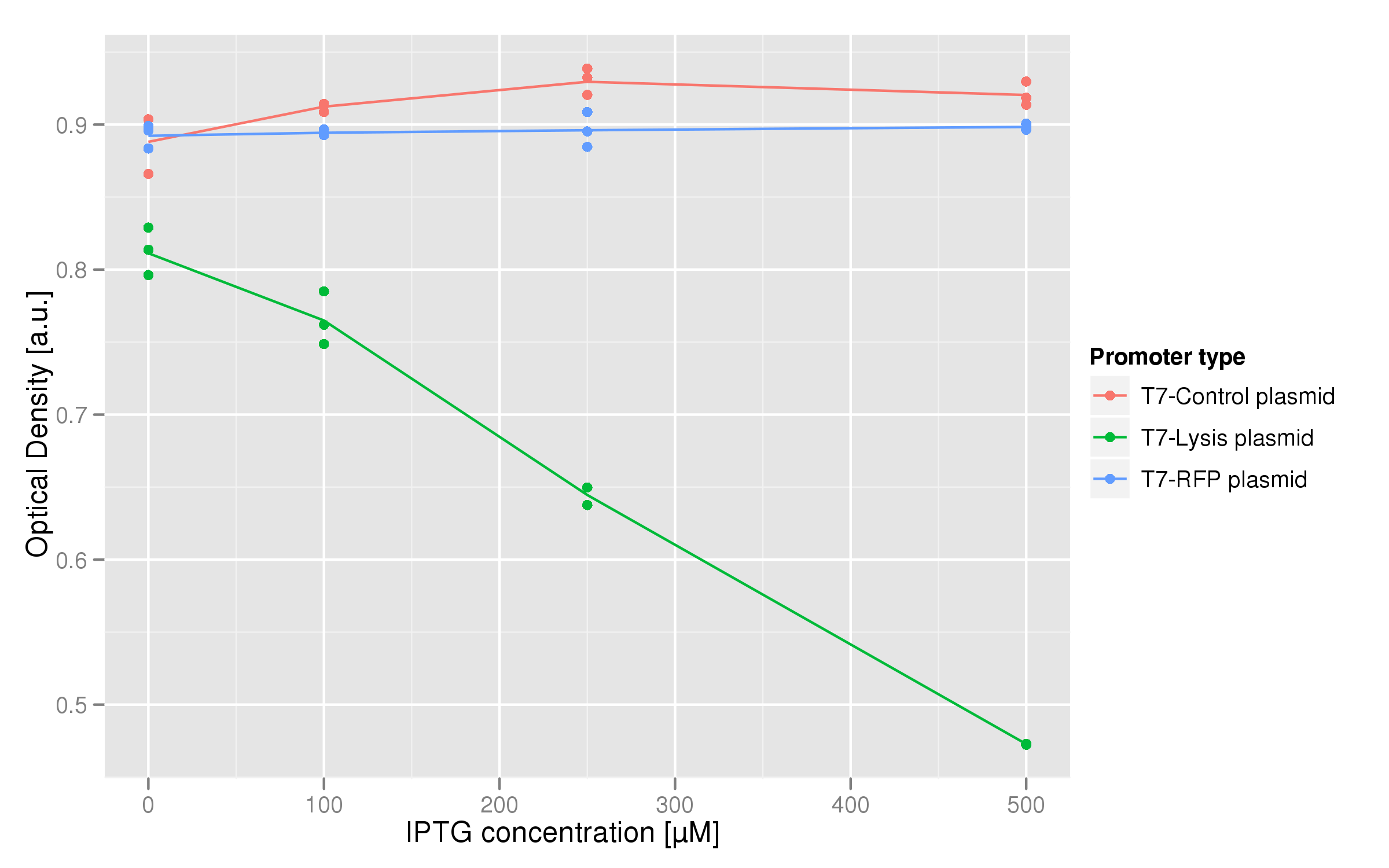
The dose-response graph above was also made using triplicate data. This time, the functional T7-lysis plasmid (C2) was tested against two negative controls: a T7-RFP plasmid (which should only fluoresce, not lyse) and a plasmid containing a non-functional lysis cassette driven by a T7 promoter (C11). The points of each curve are the unaveraged fluorescence responses (normalized by OD) over the last hour of the experiment, which we take to be a good estimate of the steady-state saturation. The C2 lysis plasmid shows strong lysing, since the fluorescence drops significantly as the IPTG dose increases. | The dose-response graph above was also made using triplicate data. This time, the functional T7-lysis plasmid (C2) was tested against two negative controls: a T7-RFP plasmid (which should only fluoresce, not lyse) and a plasmid containing a non-functional lysis cassette driven by a T7 promoter (C11). The points of each curve are the unaveraged fluorescence responses (normalized by OD) over the last hour of the experiment, which we take to be a good estimate of the steady-state saturation. The C2 lysis plasmid shows strong lysing, since the fluorescence drops significantly as the IPTG dose increases. | ||
{{:Team:EPF-Lausanne/Templates/Footer}} | {{:Team:EPF-Lausanne/Templates/Footer}} | ||
Revision as of 16:17, 21 September 2011
Lysis Selection System
Lysis selection system Main | Lysis Characterization | DNA Recovery | DNA Selection | T7 Promoter Variants
Contents |
Lysis Characterization
Introduction
We cloned the Berkeley Lysis cassette (BBa_K112808) under control of the T7 promoter into a low copy number plasmid (BBa_I739202, known as pSB3K1)
We tested lysis of cells haboring this plasmid in a platereader experiment. Lysis resulted in a drop in optical density after induction with IPTG. We observed a strong dependence on IPTG concentration.
Experimental Setup
1) We made liquid cultures of BL21 cells containing the plasmids with the lysis cassette driven by a T7 promoter. These plasmids have Kanamycin resistance, so the cultures were made using 5 mL of LB medium with 5 uL of 100 ug/uL Kanamycin and grown overnight.
2) 1:10 dilutions of cells to LB medium were made by adding 100 uL of cells to 900 uL of LB with 1 uL of Kanamycin. From this 1 mL mix, we pipetted 100 uL into the wells of a 96 well plate.
3) Each well was covered with 20 uL of mineral oil to avoid evaporation. Then we set the platereader to shake continuously and take optical density measurements at 600 nm wavelength every ten minutes.
4) We waited an hour and a half (approximately) until the cells had reached their log-linear growth phase. Then we took out the plate and added 1 uL of IPTG to each well. We made triplicate data for each concentration of IPTG (0 uL for negative control, 50 uL, 100 uL, 250 uL, and 500 uL).
5) The plate was set again into the platereader, where we ran it for 12 hours with continous shaking. Optical density measurements were made every ten minutes.
Results
Data was produced in triplicate via the 96 well plate. This graphic shows the mean over the three data points for the measurements taken every 10 minutes. The values on the y-axis are relative fluorescence units (RFU) normalized by optical density (OD), while the x-axis represents time. The curves indicate that maximal lysis is produced with 500 uM IPTG, and that lesser degrees of lysis can be obtained with smaller doses of IPTG.
The dose-response graph above was also made using triplicate data. This time, the functional T7-lysis plasmid (C2) was tested against two negative controls: a T7-RFP plasmid (which should only fluoresce, not lyse) and a plasmid containing a non-functional lysis cassette driven by a T7 promoter (C11). The points of each curve are the unaveraged fluorescence responses (normalized by OD) over the last hour of the experiment, which we take to be a good estimate of the steady-state saturation. The C2 lysis plasmid shows strong lysing, since the fluorescence drops significantly as the IPTG dose increases.
 "
"