Team:EPF-Lausanne/Our Project/T7 promoter variants
From 2011.igem.org
| Line 7: | Line 7: | ||
* [[Team:EPF-Lausanne/Our Project/T7 promoter variants/dnaselect| DNA Selection Experiment]]: Selecting the right DNA | * [[Team:EPF-Lausanne/Our Project/T7 promoter variants/dnaselect| DNA Selection Experiment]]: Selecting the right DNA | ||
* [[Team:EPF-Lausanne/Our Project/T7 promoter variants/t7make| T7 Promoter Variants]]: Making and Characterizing of T7 promoter variants for Lysis | * [[Team:EPF-Lausanne/Our Project/T7 promoter variants/t7make| T7 Promoter Variants]]: Making and Characterizing of T7 promoter variants for Lysis | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
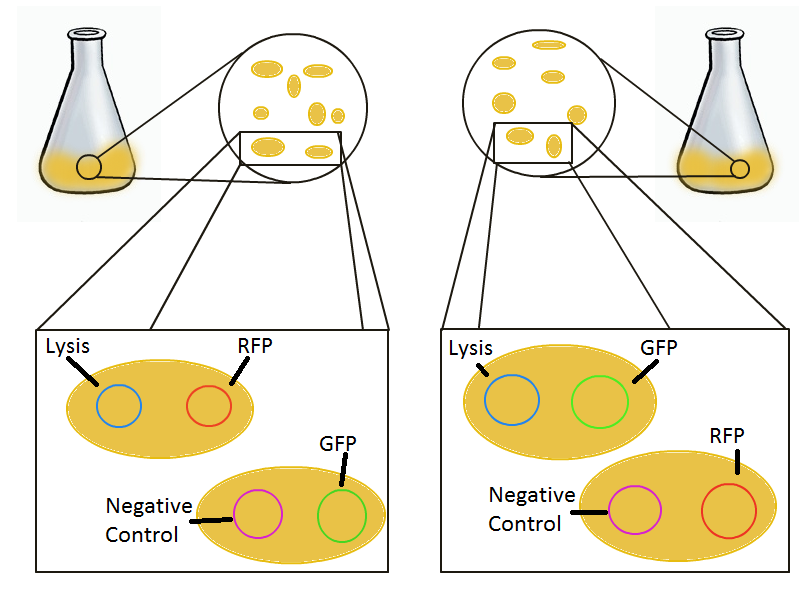
To round out the experiments for DNA recovery, it is essential that we show that the recovered DNA is in fact plasmid DNA from the relevant lysed cells. To that end, we again set up an experiment involving two flasks but this time each flask contains two cultures. For one flask, one culture is a co-transformation of a lysis plasmid and a RFP-containing plasmid and the other is a co-transformation of a negative control plasmid with a GFP-containing plasmid. In the other flask, the reverse is true: lysis is with GFP and negative control is with RFP. | To round out the experiments for DNA recovery, it is essential that we show that the recovered DNA is in fact plasmid DNA from the relevant lysed cells. To that end, we again set up an experiment involving two flasks but this time each flask contains two cultures. For one flask, one culture is a co-transformation of a lysis plasmid and a RFP-containing plasmid and the other is a co-transformation of a negative control plasmid with a GFP-containing plasmid. In the other flask, the reverse is true: lysis is with GFP and negative control is with RFP. | ||
Revision as of 10:30, 21 September 2011
Lysis Selection System
Skip straight to: |
- Platereader Lysis Experiment: Using a platereader
- DNA Recovery Experiment: Recovering DNA after lysis
- DNA Selection Experiment: Selecting the right DNA
- T7 Promoter Variants: Making and Characterizing of T7 promoter variants for Lysis
To round out the experiments for DNA recovery, it is essential that we show that the recovered DNA is in fact plasmid DNA from the relevant lysed cells. To that end, we again set up an experiment involving two flasks but this time each flask contains two cultures. For one flask, one culture is a co-transformation of a lysis plasmid and a RFP-containing plasmid and the other is a co-transformation of a negative control plasmid with a GFP-containing plasmid. In the other flask, the reverse is true: lysis is with GFP and negative control is with RFP.
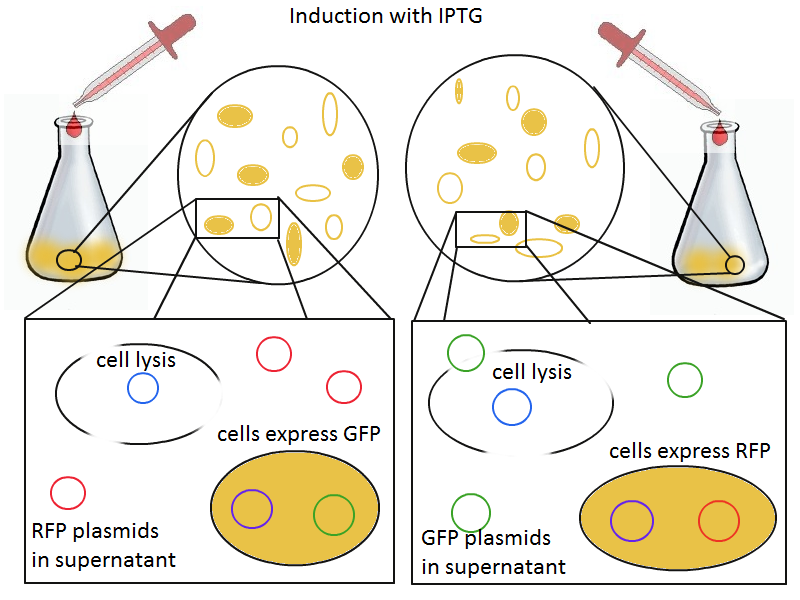
Induction with IPTG lyses the cells with the lysis cassette but leaves the cells with the negative control free to produce the red fluorescence. Collecting the supernatant and sterile filtering it reveals, by qPCR or by transforming it and counting the colonies, that the supernatant contains RFP plasmids in one flask and GFP plasmids in the other. Meanwhile, the large flask from which the supernatant samples are taken should be yellow-green and pink respectively, since the remaining cells will fluoresce according to those colors.
 "
"