Team:EPF-Lausanne/Notebook/June2011
From 2011.igem.org
(→Monday, 27 June 2011) |
(→Gibson Assembly) |
||
| (42 intermediate revisions not shown) | |||
| Line 187: | Line 187: | ||
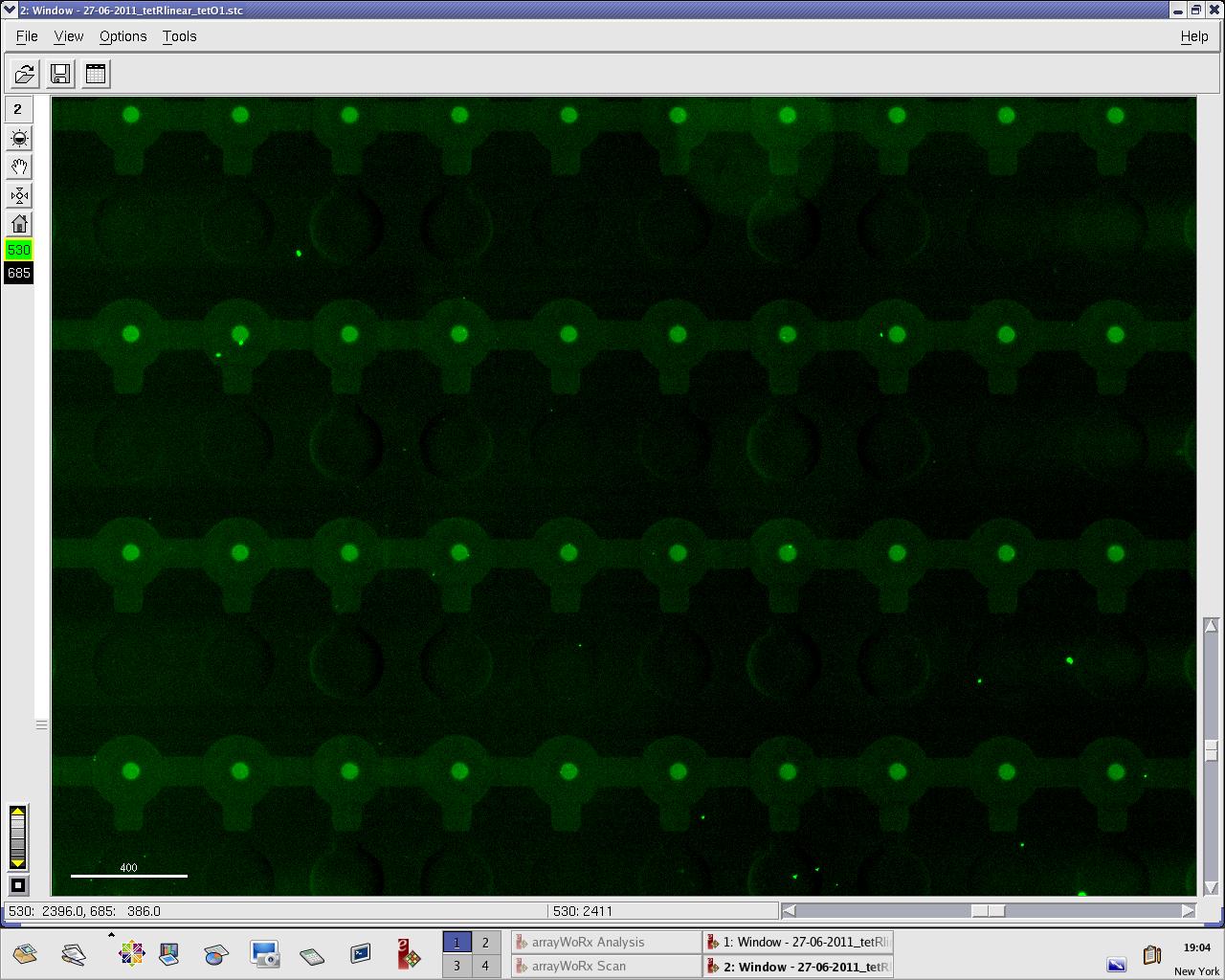
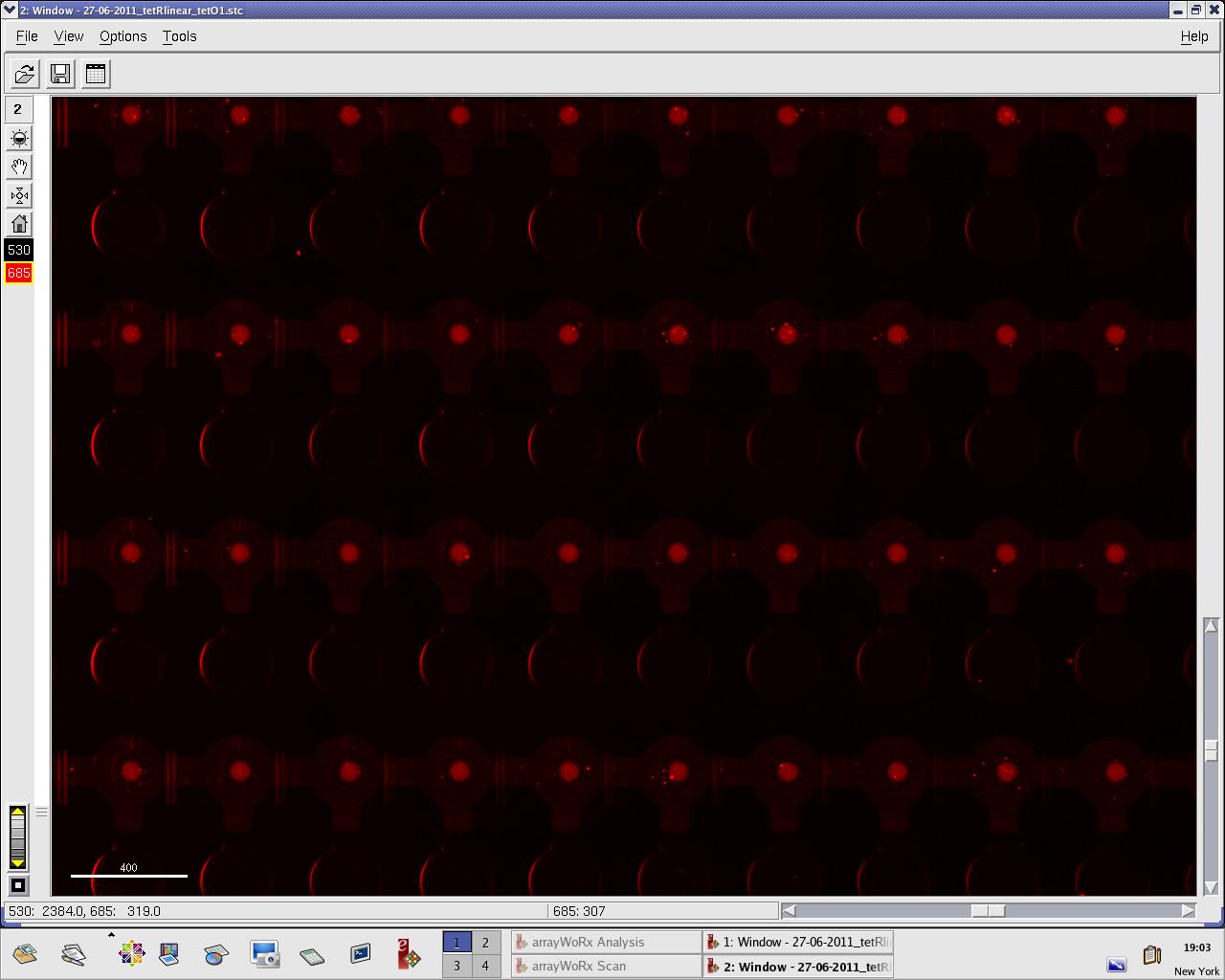
Images: Green fluorescence comes from TetR (labeled Lysines) and red fluorescence comes from DNA (cy-5 labeled). We can clearly see that the protein amount seems to be the same, whereas the DNA fluorescence is decreased. | Images: Green fluorescence comes from TetR (labeled Lysines) and red fluorescence comes from DNA (cy-5 labeled). We can clearly see that the protein amount seems to be the same, whereas the DNA fluorescence is decreased. | ||
| - | [[File:EPFL_27-06-tetR_negctrl_A488.jpg| | + | [[File:EPFL_27-06-tetR_negctrl_A488.jpg|300px|TetR fluorescence during random DNA experiment]] |
| - | [[File:EPFL_27-06-tetR_negctrl_cy5.jpg| | + | [[File:EPFL_27-06-tetR_negctrl_cy5.jpg|300px|DNA fluorescence during random DNA experiment]] |
| - | [[File:EPFL_27-06-tetR_tetO1_A488.jpg| | + | |
| - | [[File:EPFL_27-06-tetR_tetO1_cy5.jpg| | + | [[File:EPFL_27-06-tetR_tetO1_A488.jpg|300px|TetR fluorescence during target DNA experiment]] |
| + | [[File:EPFL_27-06-tetR_tetO1_cy5.jpg|300px|DNA fluorescence during target DNA experiment]] | ||
Perhaps the ITT doesn't yield a functional TetR, or we didn't have the optimal concentration of binding DNA (too much nonspecific interactions?). We'll definitely continue the MITOMI practise to find out. | Perhaps the ITT doesn't yield a functional TetR, or we didn't have the optimal concentration of binding DNA (too much nonspecific interactions?). We'll definitely continue the MITOMI practise to find out. | ||
| + | |||
| + | == Tuesday, June 28 2011 == | ||
| + | |||
| + | In order to avoid putting the lysis cassette and the LacI-TetR construct all on the same plasmid, the assembly designers chose to use a two-plasmid strategy. To get things started, we took the P23019 plasmid and the J61002 plasmid from the iGEM registry. The p23019 plasmid comes with chloramphenicol resistance, while the J61002 plasmid comes with ampicillin resistance and an RFP (red fluorescent protein) gene. | ||
| + | |||
| + | Henrike and Irina designed primers for both plasmids, with the goal of amplifying the following four parts: | ||
| + | |||
| + | |||
| + | 1) a pTet-RFP segment (820 bp) | ||
| + | *Primer 1: J61002-Ptet-RFP-r | ||
| + | *Primer 2: J61002-RFP-f | ||
| + | *DNA template: Plate 1, 18 C | ||
| + | |||
| + | |||
| + | 2) a J61002 plasmid backbone with a terminator (2334 bp) | ||
| + | *Primer 1: J61002-f | ||
| + | *Primer 2: J61002-term-r | ||
| + | *DNA template: Plate 1, 18 C | ||
| + | |||
| + | |||
| + | 3) a constitutive promoter for TetR (725 bp) | ||
| + | *Primer 1: Pconst-tetR-f | ||
| + | *Primer 2: TetR-r | ||
| + | *DNA template: TetR Repressilator plasmid | ||
| + | |||
| + | |||
| + | 4) P23019 plasmid (2242 bp) | ||
| + | *Primer 1: P23019-r | ||
| + | *Primer 2: P23019-f | ||
| + | *DNA template: Plate 4, 14E | ||
| + | |||
| + | |||
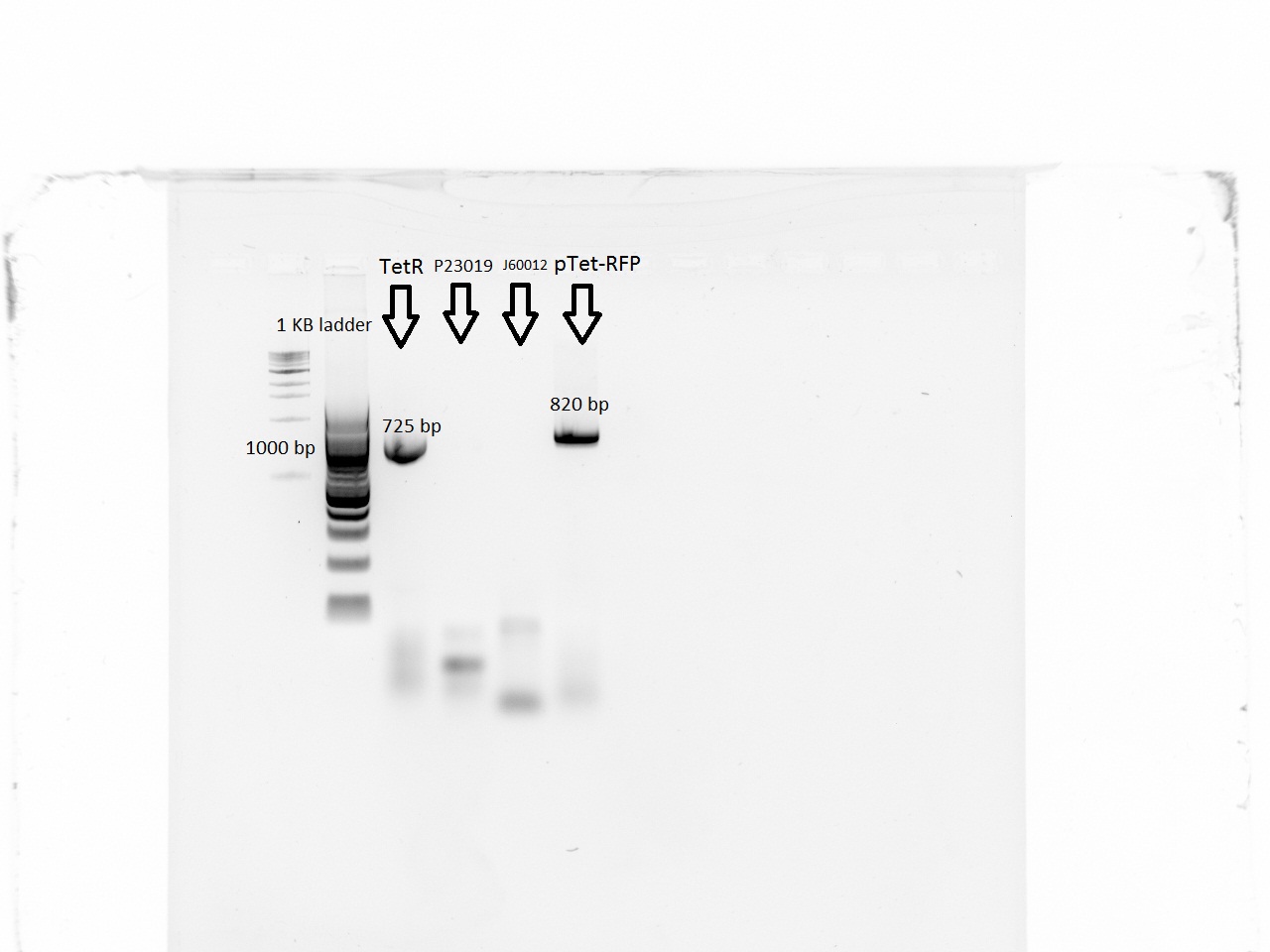
| + | With a first PCR, we were able to amplify the pTet-RFP segment and the promoter for TetR, but neither of the backbones was amplified. The iProof PCR was done using 40 seconds of elongation time at 55 C. | ||
| + | |||
| + | [[File:EPFL-28-06-pcr.jpg|300px|TetR segment and RFP-pTet segment amplified. Both backbones unamplified]] | ||
| + | |||
| + | == Wednesday, 29 June 2011 == | ||
| + | |||
| + | The mixed success of the previous day's PCR required a change in tactics. First, it was decided that the elongation time should be increased from 40 seconds to 1 minute. Second, it was deemed important to take melting temperature into consideration. The IDT (Integrated DNA Technologies) toolbox allows you to compute melting temperatures for sequences. Inserting the primer sequences yielded the following heats: | ||
| + | |||
| + | 1) P23019-r: 45.8 + 3 = 48.8 | ||
| + | |||
| + | 2) P23019-f: 60.8 + 3 = 63.8 | ||
| + | |||
| + | 3) J61002-bb-f: 52.2 + 3 = 55.2 | ||
| + | |||
| + | 4) J61002-term-r: 49.3 + 3 = 52.3 | ||
| + | |||
| + | |||
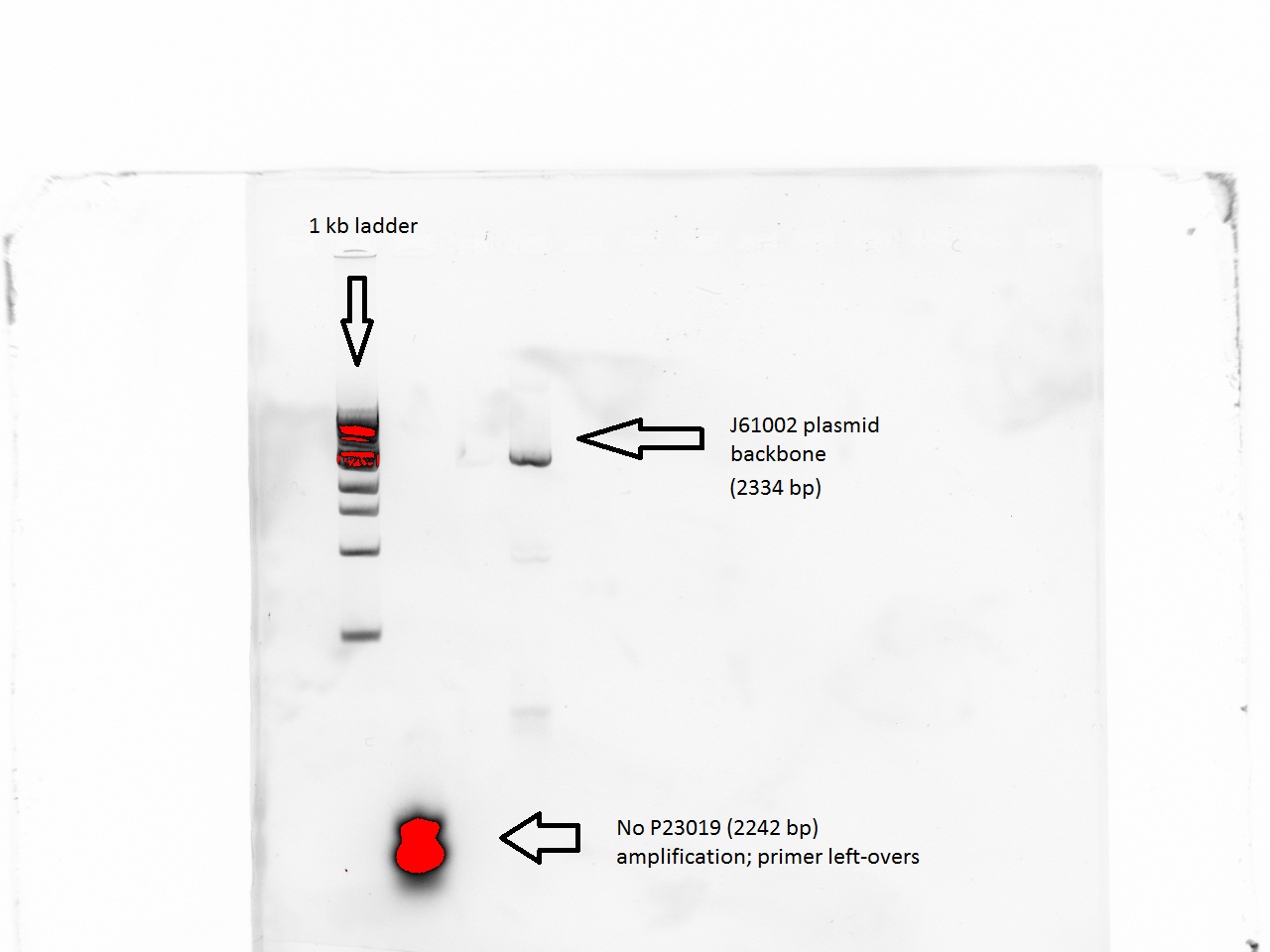
| + | The lowest temperature was 49 (48.8) so we proceeded to do a PCR with these values. The J61002 plasmid backbone was properly amplified.However, the P23019 backbone remained unamplified despite these changes. | ||
| + | |||
| + | [[File:EPFL-29-06_J6_backb.jpg|300px|J61002 backbone amplification, but no P23019]] | ||
| + | |||
| + | == Thursday, 30 June 2011 == | ||
| + | |||
| + | Alina and Lilia did MITOMI on tetR-GFP expressed from plasmid, it was loaded on chip in ITT expression mix. DNA was spotted on June 29 in different concentrations for both: consensus (tetO1) sequence and a random sequence ((-)control). | ||
| + | |||
| + | [[File:MITOMI_30juin_tetR_consensus-negctrl_check.png|600px|GFP-tetR and cy5 fluorescence]] | ||
| + | |||
| + | Images: Green fluorescence comes from GFP-TetR and red fluorescence comes from DNA (cy-5 labeled). | ||
| + | |||
| + | This experiment confirms that wtTetR is expressed and correctly folded by SP6 expression system. The TF recognizes tetO1 consensus sequence while the tested random sequence (-)control has a significantly lower binding affinity to TetR. | ||
| + | |||
| + | |||
| + | == Gibson Assembly== | ||
| + | |||
| + | |||
| + | On the Gibson assembly side of things, Vincent and Irina purified the PCR product of both the J61002 plasmid and the pTet-RFP construct using the standard PCR purification protocol. Then, using NanoDrop, they measured the concentrations of J61002 plasmid and of the RFP construct: | ||
| + | |||
| + | |||
| + | RFP (820 bp) : 28.8 ng/microL | ||
| + | |||
| + | |||
| + | J6 (2334 bp) : 26 ng/microL | ||
| + | |||
| + | |||
| + | '''Instead of following the instructions on the wiki by Nikolaus Obholzer which has the wrong formula for finding the equal molecular ratio''', we determined that the ratio ought to be 80/233 since the length of the RFP fragment is 820 bp and the length of the J6 plasmid is 2334. To make the Gibson mix, we used 2.6 microliters (80 ng % 28.8 ng/microL) of the RFP segment and 9 microliters of the plasmid (230 ng % 26 ng/microL). The assembly was done with a sample of 5 microliters taken from the 11.6 microliter mix. | ||
| + | |||
| + | We then did a transformation with these Gibson-ed plasmids and plated the cells on ampicillin plates and put them in the incubator overnight. | ||
| + | |||
| + | == Gradient PCR == | ||
| + | |||
| + | In a last attempt to try to amplify the P23019 plasmid backbone (the last piece needed to start a Gibson assembly of the TetR plasmid), Vincent tried a gradient PCR in which separate samples of the plasmid would be run at temperatures between 40 and 50 C (40, 42, 44, 46, and 48). The results showed, once again, that amplification was beyond reach. | ||
| + | |||
| + | [[File:EPFL-29-06-p23019fail.jpg|300px|GFP-tetR and cy5 fluorescence]] | ||
{{:Team:EPF-Lausanne/Templates/Footer}} | {{:Team:EPF-Lausanne/Templates/Footer}} | ||
Latest revision as of 16:51, 9 July 2011
Notebook: June 2011
Contents |
Friday, 3 June 2011
Lysis device sequences were received from Microsynth. Only two reactions worked: those containing primers 1289F and 1333R.
Output (For the reverse primers, the reverse complement sequence is shown):
>1556291 30F
NNNNN
>1556292 440F
NNNNN
>1556293 947F
NNNNN
>1556294 1289F
NNNCTTCGGNGGGCCTTTCTGCGTTNNNATACTANTAGCGGCCGCTGCAGTCCGGCAAAAAAGGGCAAGGTGTCACCACCCTGCCCTTTTTCTTTAAAAC
CGAAAAGATTACTTCGCGTTATGCAGGCTTCCTCGCTCACTGACTCGCTGCGCTCGGTCGTTCGGCTGCGGCGAGCGGTATCAGCTCACTCAAAGGCGGT
AATACGGTTATCCACAGAATCAGGGGATAACGCAGGAAAGAACATGTGAGCAAAAGGCCAGCAAAAGGCCAGGAACCGTAAAAAGGCCGCGTTGCTGGCG
TTTTTCCACAGGCTCCGCCCCCCTGACGAGCATCACAAAAATCGACGCTCAAGTCAGAGGTGGCGAAACCCGACAGGACTATAAAGATACCAGGCGTTTC
CCCCTGGAAGCTCCCTCGTGCGCTCTCCTGTTCCGACCCTGCCGCTTACCGGATACCTGTCCGCCTTTCTCCCTTCGGGAAGCGTGGCGCTTTCTCATAG
CTCACGCTGTAGGTATCTCAGTTCGGTGTAGGTCGTTCGCTCCAAGCTGGGCTGTGTGCACGAACCCCCCGTTCAGCCCGACCGCTGCGCCTTATCCGGT
AACTATCGTCTTGAGTCCAACCCGGTAAGACACGACTTATCGCCACTGGCAGCAGCCACTGGTAACAGGATTAGCAGAGCGAGGTATGTAGGCGGTGCTA
CAGAGTTCTTGAAGTGGTGGCCTAACTACGGCTACACTAGAAGAACAGTATTTGGTATCTGCGCTCTGCTGAAGCCAGTTACCTTCGGAANAAGAGTTGG
TAGCTCTTGATCCGGCAAACAAACCACCGCTGGTAGCGGTGGTTTTTTTGTTTGCAAGCAGCAGATTACGCGCAGAAAANAAGGATCTCAAGAAGATCCT
TTGATCTTTTCTACGGGGTCTGACGCTCAGTGGAACGAANACTCACGTTAAGGGATTTTGGTCATGAGATTATCAAAAAGGATCTTCACCTAGATCCTTT
TAAATTAAAAATGAAGTTTTANATCAATCTAAAGTATATATGANTAAACTTGGTCTGANAGCTCGAGATTCTCATGTTTGANAGCTTATCATCGATAAGC
TTTAATGNGGTAGTTTATCNNAGTTAAATTGCTAACGCAGTCAGGNNCCGNN
>1556295 816R
NNNNN
>1556296 1333R
TNCNTTNCTAGAGGGNCAAGCAATGCTTGCCTTGAATANNAACTTTTGAATANNGATTCAGGAGGTACTAGAATGGCTTCCAAGGTGTACGACCCCGAGC
AACGCAAACGCATGATCACTGGGCCTCAGTGGTGGGCTCGCTGCAAGCAAATGAACGTGCTGGACTCCTTCATCAACTACTATGATTCCGAGAAGCACGC
CGAGAACGCCGTGATTTTTNTGCATGGTAACGCTGCCTCCAGCTACCTGTGGAGGCACGTCGTGCCTCACATCGAGCCCGTGGCTAGATGCATCATCCCT
GATCTGATCGGAATGGGTAAGTCCGGCAAGAGCGGGAATGGCTCATATCGCCTCCTGGATCACTACAAGTACCTCACCGCTTGGTTCGAGCTGCTGAACC
TTCCAAAGAAAATCATCTTTGTGGGCCACGACTGGGGGGCTTGTCTGGCCTTTCACTACTCCTACGAGCACCAAGACAAGATCAAGGCCATCGTCCATGC
TGAGAGTGTCGTGGACGTGATCGAGTCCTGGGACGAGTGGCCTGACATCGAGGAGGATATCGCCCTGATCAAGAGCGAAGAGGGCGAGAAAATGGTGCTT
GAGAATAACTTCTTCGTCGAGACCATGCTCCCAAGCAAGATCATGCGGAAACTGGAGCCTGAGGAGTTCGCTGCCTACCTGGAGCCATTCAAGGAGAAGG
GCGAGGTTAGACGGCCTACCCTCTCCTGGCCTCGCGAGATCCCTCTCGTTAAGGGAGGCAAGCCCGACGTCGTCCAGATTGTCCGCAACTACAACGCCTA
CCTTCGGGCCAGCGACGATCTGCCTAAGATGTTCATCGAGTCCGACCCTGGGTTCTTTTCCAACGCTATTGTCGAGGGAGCTAAGAAGTTCCCTAACACC
GAGTTCGTGAAGGTGAAGGGCCTCCACTTCAGCCAGGAGGACGCTCCAGATGAAATGGGTAAGTACATCAAGAGCTTCGTGGAGCGCGTGCTGAAGANNN
GAGCAGTAAACTAGAGCCAGGCATCAAATAAAACGAAAGGNTCAGTCGAAAGACTGGGCCTTTCGTTTNNNN
>1556297 1734R
NNNNN
Monday, 6 June 2011
Irina and Nadine selected the 2 plasmid backbones we will use: J23019 and J61002 (check the parts registry). They have 2 different origin of replication sites and should both have 15-20 copy numbers. Interesting also, they both contain RFP and pTet. We already have them in the freezers, now let's focus on primers design!
Wednesday, 8 June 2011
Lilia and Douglas miniprep'ed the cultures containing the lysis cassette, and sent the plasmids for sequencing.
Three colonies were miniprep'ed: two containing the T4 variant, one containing the lambda variant. This yielded solutions with plasmid concentrations of 221.9 ng/µl for T4 colony 1, 225.5 ng/µl for T4 colony 2, and 57.0 ng/µl for lambda colony. Only T4 colony 1 was sent for sequencing, using the primer solutions prepared on 31 May 2011. Results should be in on Friday.
Friday, 10 June 2011
Sequencing results from Microsynth for the lysis cassette (Biobrick K112808) have arrived. The sequence is entirely covered by the seven reactions, and all but a few bases match. As far as I can tell, this biobrick is valid! (Doug)
The results of a blast, individually querying each of the sequences against the official K112808 sequence are available here: BLAST results.
Sequencing results, for each primer (again, reverse complements shown here for the reverse primers --- those denoted by an "R"):
>1558332 30F NGNNNTCATTTGGTGTTCAGATCGCTTGTTCAAGATAACGCTACCGGGAAGGTTCTTGCTTCCCGGGTAGCTGTCGTAATTCTTTTGTTTATAATGGCGA TTGTTTGGTATAGGGGAGATAGTTTCTTTGAGTACTATAAGCAATCAAAGTATGAAACATACAGTGAAATTATTGAAAAGGAAAGAACTGCACGCTTTGA ATCTGTCGCCCTGGAACAACTCCAGATAGTTCATATATCATCTGAGGCAGACTTTAGTGCGGTGTATTCTTTCCGCCCTAAAAACTTAAACTATTTTGTT GATATTATAGCATACGAAGGAAAATTACCTTCAACAATAAGTGAAAAATCACTTGGAGGATATCCTGTTGATAAAACTATGGATGAATATACAGTTCATT TAAATGGACGTCATTATTATTCCAACTCAAAATTTGCTTTTTTACCAACTAAAAAGCCTACTCCCGAAATAAACTACATGTACAGTTGTCCATATTTTAA TTTGGATAATATCTATGCTGGAACGATAACCATGTACTGGTATAGAAATGATCATATAAGTAATGACCGCCTTGAATCAATATGTGCTCAGGCGGCCAGA ATATTAGGAAGGGCTAAATAATTAACTAGAATACTTAGGAGGTATTATGAATATATTTGAAATGTTACGTATAGATGAAGGTCTTAGACTTAAAATCTAT AAAGACACAGAAGGCTATTACACTATTGGCATCGGTCATTTGCTTACAAAAAGTCCATCACTTAATGCTGCTAAATCTGAATTAGATAAAGCTATTGGGC GTAATTGCAATGGTGTAATTACAAAAGATGAGGCTGAAAAACTCTTTAATCAGGATGTTGATGCTGCTGTTCGCGGAATCCTGAGAAATGCTAAATTAAA ACCGGTTTATGATTCTCTTGATGCGGTTCGTCGCTGTGCATTGATTAATATGGTTTTCCAAATGGGANAAACCGGTGTGGCAGGATTTACTAACTCTTTA CGTATGCTTCAACAAAAACGCTGGGATGAAGCAGCAGTTAACTTAGCTAANAGTAGATGGNNTAATCAAACNCCTAATCNNNNAAAACGAGTCATTACAA CGTTTANAACTGGNNN >1558333 440F NNNATTCCNACTCAAATTTGCTTTTTTACCAACTAAAAAGCCTACTCCCGAAATAAACTACATGTACAGTTGTCCATATTTTAATTTGGATAATATCTAT GCTGGAACGATAACCATGTACTGGTATAGAAATGATCATATAAGTAATGACCGCCTTGAATCAATATGTGCTCAGGCGGCCAGAATATTAGGAAGGGCTA AATAATTAACTAGAATACTTAGGAGGTATTATGAATATATTTGAAATGTTACGTATAGATGAAGGTCTTAGACTTAAAATCTATAAAGACACAGAAGGCT ATTACACTATTGGCATCGGTCATTTGCTTACAAAAAGTCCATCACTTAATGCTGCTAAATCTGAATTAGATAAAGCTATTGGGCGTAATTGCAATGGTGT AATTACAAAAGATGAGGCTGAAAAACTCTTTAATCAGGATGTTGATGCTGCTGTTCGCGGAATCCTGAGAAATGCTAAATTAAAACCGGTTTATGATTCT CTTGATGCGGTTCGTCGCTGTGCATTGATTAATATGGTTTTCCAAATGGGAGAAACCGGTGTGGCAGGATTTACTAACTCTTTACGTATGCTTCAACAAA AACGCTGGGATGAAGCAGCAGTTAACTTAGCTAAAAGTAGATGGTATAATCAAACACCTAATCGCGCAAAACGAGTCATTACAACGTTTAGAACTGGCAC TTGGGACGCGTATAANAATCTATAAAGCACTAGAGCCAGGCATCAAATAAAACGAAAGGCTCAGTCGAAAGACTGGGCCTTTCGTTTTATCTGTTGTTTG TCGGTGAACGCTCTCTACTAGAGTCACACTGGCTCACCTTCGGGTGGGCCTTTCTGCGTTTATATACTAGAGTTGACAGCTAGCTCAGTCCTAGGGACTA TGCTAGCTACTAGAGATAGGAGGCCTTTATGGCCTTAAAAGCAACAGCACTTTTTGCCATGCTAGGATTGTCATTTGTTTTATCTCCATCGATTGAAGCG AATGTCNATCCTCATTTTGATAAATTTATGGAATCTGGNNTTAGGCACGTTTATNTGCTTTTTGAAAANAAAANCGTANAATCNNCTGAACAATTCTATA GTTTTANNNNAACGACCTATAAAANTGACCCNNGN >1558334 947F NNTTGNNGCGGTTCGTCGCTGTGCATTGATTAATATGGTTTTCCAAATGGGAGAAACCGGTGTGGCAGGATTTACTAACTCTTTACGTATGCTTCAACAA AAACGCTGGGATGAAGCAGCAGTTAACTTAGCTAAAAGTAGATGGTATAATCAAACACCTAATCGCGCAAAACGAGTCATTACAACGTTTAGAACTGGCA CTTGGGACGCGTATAAAAATCTATAAAGCACTAGAGCCAGGCATCAAATAAAACGAAAGGCTCAGTCGAAAGACTGGGCCTTTCGTTTTATCTGTTGTTT GTCGGTGAACGCTCTCTACTAGAGTCACACTGGCTCACCTTCGGGTGGGCCTTTCTGCGTTTATATACTAGAGTTGACAGCTAGCTCAGTCCTAGGGACT ATGCTAGCTACTAGAGATAGGAGGCCTTTATGGCCTTAAAAGCAACAGCACTTTTTGCCATGCTAGGATTGTCATTTGTTTTATCTCCATCGATTGAAGC GAATGTCGATCCTCATTTTGATAAATTTATGGAATCTGGTATTAGGCACGTTTATATGCTTTTTGAAAATAAAAGCGTAGAATCGTCTGAACAATTCTAT AGTTTTATGAGAACGACCTATAAAAATGACCCGTGCTCTTCTGATTTTGAATGTATAGAGCGAGGCGCGGAGATGGCACAATCATACGCTAGAATTATGA ACATTAAATTGGAGACTGAATGANATACTAGAGCCGGCTTATCGGTCAGTTTCACCTGATTTACGTAAAAACCCGCTTCGGCGGGTTTTTGCTTTTGGAG GGGCAGAAAGATGAATGACTGTCCACGACGCTATACCCAAAAGAAATACTAGTCTGCAGGCTTCCTCGCTCACTGACTCGCTGCGCTCGGTCGTTCGGCT GCGGCGAGCGGTATCAGCTCACTCAAAGGCGGTAATACGGTTATCCACAGAATCAGGGGATAACGCAGGAAAGAACATGTGAGCAAAAGGCCAGCAAAAG GCCAGGAACCGTAAAAAGGCCGCGTTGCTGGCGTTTTTCCNCAGGCTCCGCCCCCCTGACGANCATCNCAAAAATCGACGCTCAAGTCANANGNN >1558335 1289F NNCTTCGGNGGGCTTTCTGNGTTTATATANTAGAGTTGACAGCTAGCTCAGTCCTAGGGACTATGCTAGCTACTAGAGATAGGAGGCCTTTATGGCCTTA AAAGCAACAGCACTTTTTGCCATGCTAGGATTGTCATTTGTTTTATCTCCATCGATTGAAGCGAATGTCGATCCTCATTTTGATAAATTTATGGAATCTG GTATTAGGCACGTTTATATGCTTTTTGAAAATAAAAGCGTAGAATCGTCTGAACAATTCTATAGTTTTATGAGAACGACCTATAAAAATGACCCGTGCTC TTCTGATTTTGAATGTATAGAGCGAGGCGCGGAGATGGCACAATCATACGCTAGAATTATGAACATTAAATTGGAGACTGAATGAAATACTAGAGCCGGC TTATCGGTCAGTTTCACCTGATTTACGTAAAAACCCGCTTCGGCGGGTTTTTGCTTTTGGAGGGGCAGAAAGATGAATGACTGTCCACGACGCTATACCC AAAAGAAATACTAGTCTGCAGGCTTCCTCGCTCACTGACTCGCTGCGCTCGGTCGTTCGGCTGCGGCGAGCGGTATCAGCTCACTCAAAGGCGGTAATAC GGTTATCCACAGAATCAGGGGATAACGCAGGAAAGAACATGTGAGCAAAAGGCCAGCAAAAGGCCAGGAACCGTAAAAAGGCCGCGTTGCTGGCGTTTTT CCACAGGCTCCGCCCCCCTGACGAGCATCACAAAAATCGACGCTCAAGTCAGAGGTGGCGAAACCCGACAGGACTATAAAGATACCAGGCGTTTCCCCCT GGAAGCTCCCTCGTGCGCTCTCCTGTTCCGACCCTGCCGCTTACCGGATACCTGTCCGCCTTTCTCCCTTCGGGAAGCGTGGCGCTTTCTCATAGCTCAC GCTGTAGGTATCTCAGTTCGGTGTAGGTCGTTCGCTCCAAGCTGGGCTGTGTGCACGAACCCCCCGTTCAGCCCGACCGCTGCGCCTTATCCGGTAACTA TCGTCTTGAGTCCAACCCGGTAAGACACGACTTATCGCCACTGGCAGCAGCCACTGGTAANAGGATTAGCANNNNNNGGTATGTAGGNNNNNCTACAGAN TTCTTGAAGTGGNGGCCTAACTACGNNTAN >1558336 816R NTTCAGNNTNTTTTACTTTCACCAGCGTTTNTGGGNGANNAAAAACAGGAAGGCAAAATGCCGCAAAAAAGGGAATAAGGGNGACACGGAAATGTTGAAT ACTCATACTCTTCCTTTTTCAATATTATTGAAGCATTTNTCAGGGTTATTGTNTCATGAGCGGATACATATTTGAATGTATTTAGAAAAATAAACAAATA GNGGTTCCGCGCACATTTCCCCGAAAAGTGCCACCTGACGTCTAAGAAACCATTATTATCATGACATTAACCTATAAAAATAGGCGTATCACGAGGCAGA ATNTCAGATAAAAAAAATCCTTAGCTTTCGCTAAGGATGATTTCTGGAATTCGCGGCCGCTTCTAGACTTAAAAGGAGGGTCTATGGCAGCACCTAGAAT ATCATNTTCGCCCTCTGATATTCTATTTGGTGTTCTAGATCGCTTGTTCAAAGATAACGCTACCGGGAAGGTTCTTGCTTCCCGGGTAGCTGTCGTAATT CTTTTGTTTATAATGGCGATTGTTTGGTATAGGGGAGATAGTTTCTTTGAGTACTATAAGCAATCAAAGTATGAAACATACAGTGAAATTATTGAAAAGG AAAGAACTGCACGCTTTGAATCTGTCGCCCTGGAACAACTCCAGATAGTTCATATATCATCTGAGGCAGACTTTAGTGCGGTGTATTCTTTCCGCCCTAA AAACTTAAACTATTTTGTTGATATTATAGCATACGAAGGAAAATTACCTTCAACAATAAGTGAAAAATCACTTGGAGGATATCCTGTTGATAAAACTATG GATGAATATACAGTTCATTTAAATGGACGTCATTATTATTCCAACTCAAAATTTGCTTTTTTACCAACTAAAAAGCCTACTCCCGAAATAAACTACATGT ACAGTTGTCCATATTTTAATTTGGATAATATCTATGCTGGAACGATAACCATGTACTGGTATAGAAATGATCATATAAGTAATGACCGCCTTGAATCAAT ATGTGCTCAGGCGGCCAGAATATTAGGAAGGGCTAAATAATTAACTAGAATACTTAGGAGGTATTATGAATATATTTGAAATGTTACGTATAGATNAAGG TCTAGACTAAANNCN >1558337 1333R CCGGGAAGGTTNTTNNNTCCCGGGTAGNNNNCGTAATNNTTNNNTNNNAATGNNGATTGTTTGTNTAGGGGAGATAGTTTNTTTGAGTACNATAAGCAAT CAAAGTNTGAAACATCCAGNGAAATTATTGAAAAGGAAANAACTGCACNCTTTGAATCTNTNGCCCTGGAACAACTCCAGATAGTTCATATNTCATNTGA GGCAGACTTTAGTGCGGTGTATTCTNTCCGCCCTAAAAACTTAAACTATTTTGTTGATATTATAGCATACGAAGGAAAATTACCTTCAACAATAAGTGAA AAATCACTTGGAGGATATCCTGTTGATAAAACTATGGATGAATATACAGTTCATTTAAATGGACGTCATTATTATTCCAACTCAAAATTTGCTTTTTTAC CAACTAAAAAGCCTACTCCCGAAATAAACTACATGTACAGTTGTCCATATTTTAATTTGGATAATATCTATGCTGGAACGATAACCATGTACTGGTATAG AAATGATCATATAAGTAATGACCGCCTTGAATCAATATGTGCTCAGGCGGCCAGAATATTAGGAAGGGCTAAATAATTAACTAGAATACTTAGGAGGTAT TATGAATATATTTGAAATGTTACGTATAGATGAAGGTCTTAGACTTAAAATCTATAAAGACACAGAAGGCTATTACACTATTGGCATCGGTCATTTGCTT ACAAAAAGTCCATCACTTAATGCTGCTAAATCTGAATTAGATAAAGCTATTGGGCGTAATTGCAATGGTGTAATTACAAAAGATGAGGCTGAAAAACTCT TTAATCAGGATGTTGATGCTGCTGTTCGCGGAATCCTGAGAAATGCTAAATTAAAACCGGTTTATGATTCTCTTGATGCGGTTCGTCGCTGTGCATTGAT TAATATGGTTTTCCAAATGGGAGAAACCGGTGTGGCAGGATTTACTAACTCTTTACGTATGCTTCAACAAAAACGCTGGGATGAAGCAGCAGTTAACTTA GCTAAAAGTAGATGGTATAATCAAACACCTAATCGCGCAAAACGAGTCATTACAACGTTTAGAACTGGCACTTGGGACGCGTATAAAAATCTATAAAGCA CTAGAGCCAGGCATCAAATAAAACGAAAGGCTCAGTCGAAAGACTGGGCCNTTCGNTTNN >1558338 1734R TCCAACTCAAAATTTNNTTTNTTNCCAACTAAAAANNCTNNTCCCGAAATAAACTNNANGNNNAGTNNTCCANNTNTTAATTTGGATAANNNNTNNGCTG GAACGATAACCATGTACTGGTATAGAAATGATCANNNAAGTAATGACCNCCTTGAATCAATATGTGNTCAGGNGNCCAGAATATTAGGAAGGGCTAAATA ATTAACTAGAATACTTAGGAGGTATTATGAATATATTTGAAATGTTACGTATAGATGAAGGTNTTAGACTTAAAATCTATAAAGACACAGAAGGCTATTA CACTATTGGCATCGGTCATTTGCTTACAAAAAGTCCATCACTTAATGCTGCTAAATCTGAATTAGATAAAGCTATTGGGCGTAATTGCAATGGTGTAATT ACAAAAGATGAGGCTGAAAAACTCTTTAATCAGGATGTTGATGCTGCTGTTCGCGGAATCCTGAGAAATGCTAAATTAAAACCGGTTTATGATTCTCTTG ATGCGGTTCGTCGCTGTGCATTGATTAATATGGTTTTCCAAATGGGAGAAACCGGTGTGGCAGGATTTACTAACTCTTTACGTATGCTTCAACAAAAACG CTGGGATGAAGCAGCAGTTAACTTAGCTAAAAGTAGATGGTATAATCAAACACCTAATCGCGCAAAACGAGTCATTACAACGTTTAGAACTGGCACTTGG GACGCGTATAAAAATCTATAAAGCACTAGAGCCAGGCATCAAATAAAACGAAAGGCTCAGTCGAAAGACTGGGCCTTTCGTTTTATCTGTTGTTTGTCGG TGAACGCTCTCTACTAGAGTCACACTGGCTCACCTTCGGGTGGGCCTTTCTGCGTTTATATACTAGAGTTGACAGCTAGCTCAGTCCTAGGGACTATGCT AGCTACTAGAGATAGGAGGCCTTTATGGCCTTAAAAGCAACAGCACTTTTTGCCATGCTAGGATTGTCATTTGTTTTATCTCCATCGATTGAAGCGAATG TCGATCCTCATTTTGATAAATTTATGGAATCTGGTATTAGGCACGTTTATATGCTTTTTGAAAATAAAAGCGTAGAATCGTCTGAACAATTCTATAGTTT TATGAGAACGACCTATAAAAATGACCCGTGCTCTTCTGATTTTGAATGTATAGAGCGAGGCGCGGAGANNNCACAN
Tuesday, 21 June 2011
With help from Henrike, Douglas checked the mistakes in the K112808 (lysis cassette) sequence. On the entire sequence, only 7 errors occurred in several reactions, and are therefore likely to be actual errors in the DNA. They occur in positions (with regard to the original sequence in the registry):
- 70: CTC codon substituded for CTA. Both code for leu, so that position is still valid
The following are missing bases:
- 677 and 684: lie in the junction between sub-bricks K112805 and K112806. Still valid.
- 1199: lies in the junction between K112806 and B0010. Still valid.
- 1387: second base of K112807, in the non-coding region. Still valid.
- 1698: lies in the junction between K112807 and B0010. Still valid.
- 1701: beginning of B0010, in the non-coding region. Still valid.
In conclusion, there is one codon substitution, and six missing bases that all lie within the junction between two bricks or the non-coding region of a brick. Experimentally, the brick does lyse cells in culture. Therefore, the sequence appears valid.
Monday, 27 June 2011
We practised MITOMI with TetR linear template and random DNA sequence + target DNA sequence. We performed ITT (in vitro transcription/translation) on TetR linear template before adding it to the chip. However, the results are disappointing: we have more DNA binding for the random sequence (1000 RFU change) than for the target sequence (500 RFU)...
Images: Green fluorescence comes from TetR (labeled Lysines) and red fluorescence comes from DNA (cy-5 labeled). We can clearly see that the protein amount seems to be the same, whereas the DNA fluorescence is decreased.
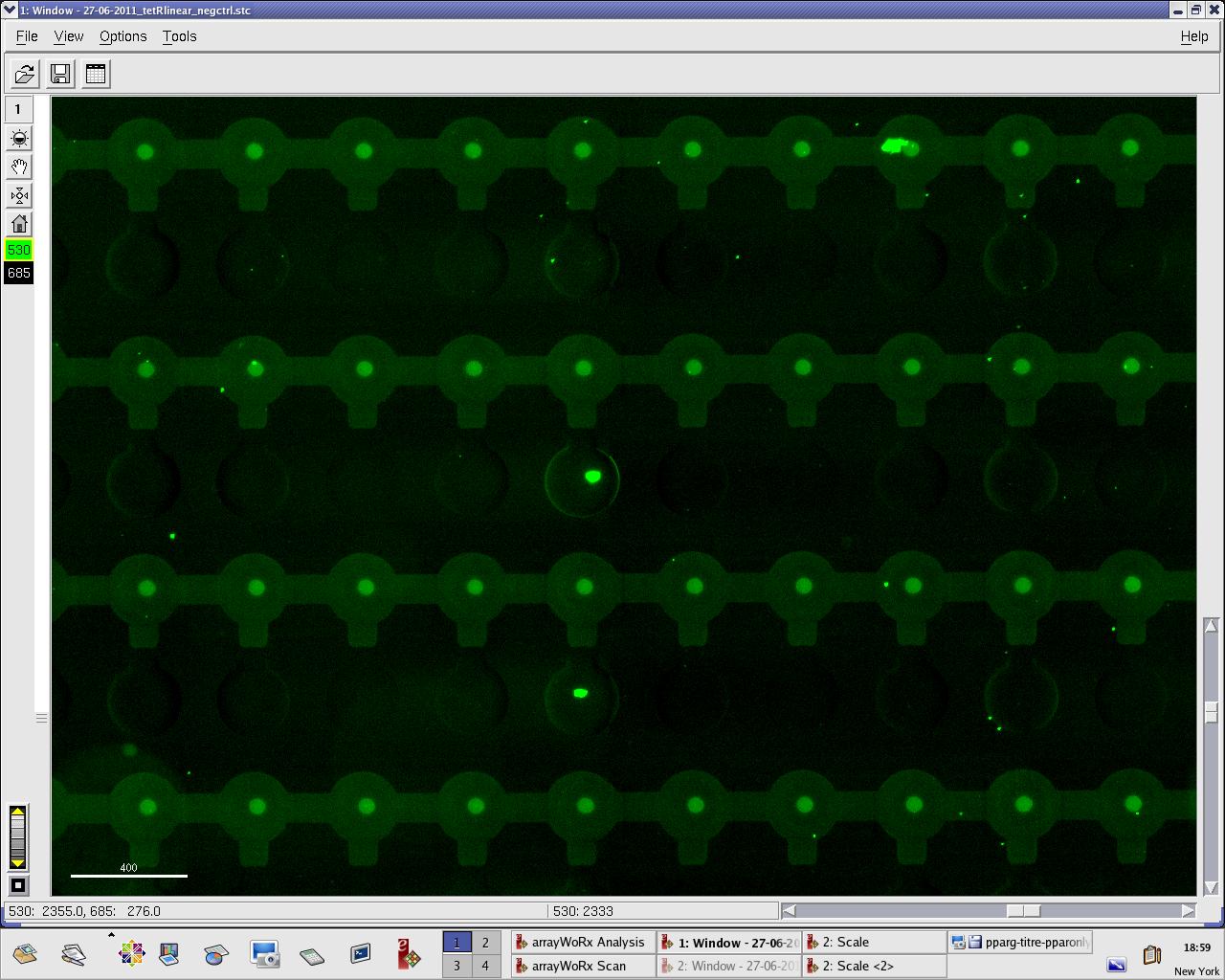
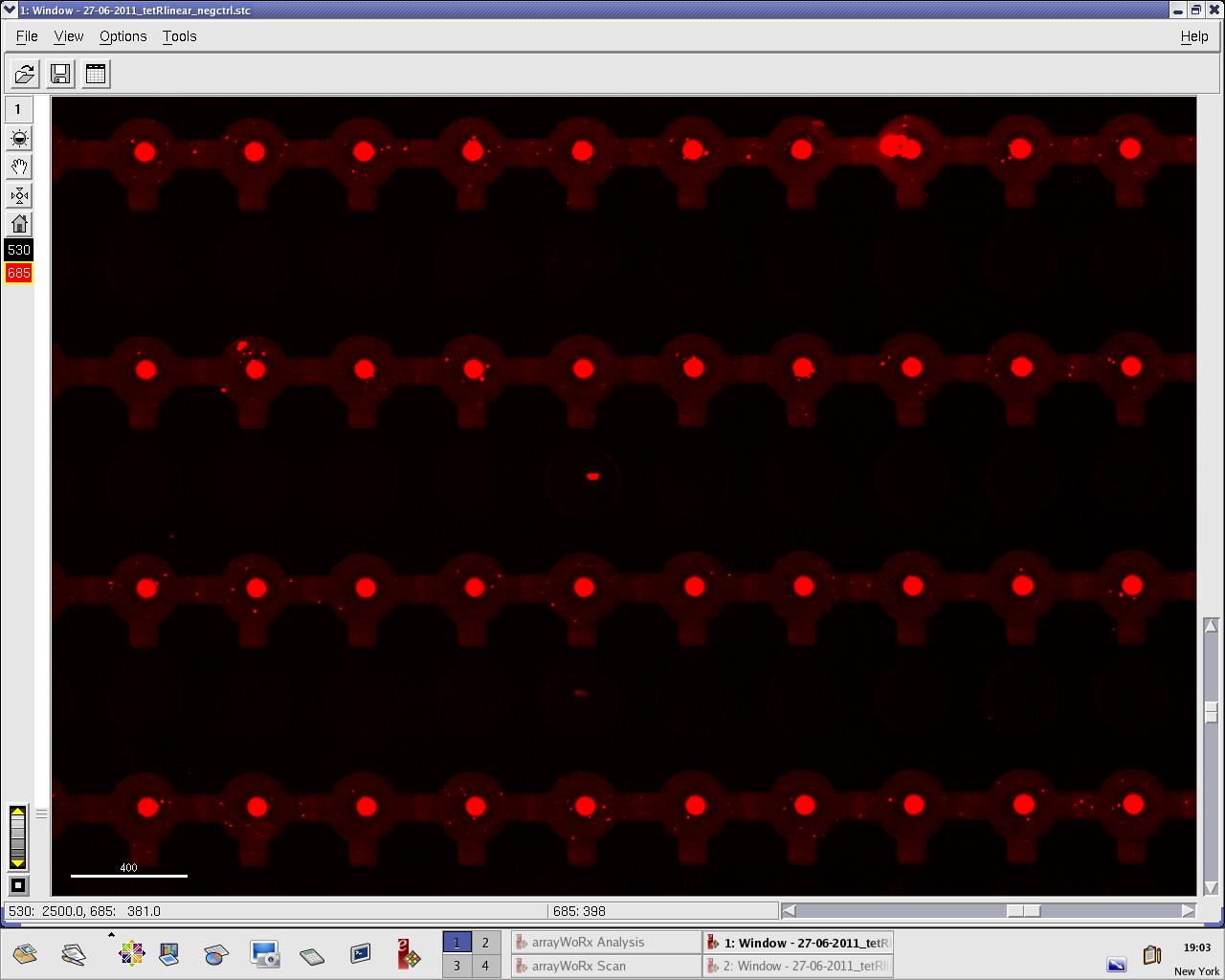
Perhaps the ITT doesn't yield a functional TetR, or we didn't have the optimal concentration of binding DNA (too much nonspecific interactions?). We'll definitely continue the MITOMI practise to find out.
Tuesday, June 28 2011
In order to avoid putting the lysis cassette and the LacI-TetR construct all on the same plasmid, the assembly designers chose to use a two-plasmid strategy. To get things started, we took the P23019 plasmid and the J61002 plasmid from the iGEM registry. The p23019 plasmid comes with chloramphenicol resistance, while the J61002 plasmid comes with ampicillin resistance and an RFP (red fluorescent protein) gene.
Henrike and Irina designed primers for both plasmids, with the goal of amplifying the following four parts:
1) a pTet-RFP segment (820 bp)
- Primer 1: J61002-Ptet-RFP-r
- Primer 2: J61002-RFP-f
- DNA template: Plate 1, 18 C
2) a J61002 plasmid backbone with a terminator (2334 bp)
- Primer 1: J61002-f
- Primer 2: J61002-term-r
- DNA template: Plate 1, 18 C
3) a constitutive promoter for TetR (725 bp)
- Primer 1: Pconst-tetR-f
- Primer 2: TetR-r
- DNA template: TetR Repressilator plasmid
4) P23019 plasmid (2242 bp)
- Primer 1: P23019-r
- Primer 2: P23019-f
- DNA template: Plate 4, 14E
With a first PCR, we were able to amplify the pTet-RFP segment and the promoter for TetR, but neither of the backbones was amplified. The iProof PCR was done using 40 seconds of elongation time at 55 C.
Wednesday, 29 June 2011
The mixed success of the previous day's PCR required a change in tactics. First, it was decided that the elongation time should be increased from 40 seconds to 1 minute. Second, it was deemed important to take melting temperature into consideration. The IDT (Integrated DNA Technologies) toolbox allows you to compute melting temperatures for sequences. Inserting the primer sequences yielded the following heats:
1) P23019-r: 45.8 + 3 = 48.8
2) P23019-f: 60.8 + 3 = 63.8
3) J61002-bb-f: 52.2 + 3 = 55.2
4) J61002-term-r: 49.3 + 3 = 52.3
The lowest temperature was 49 (48.8) so we proceeded to do a PCR with these values. The J61002 plasmid backbone was properly amplified.However, the P23019 backbone remained unamplified despite these changes.
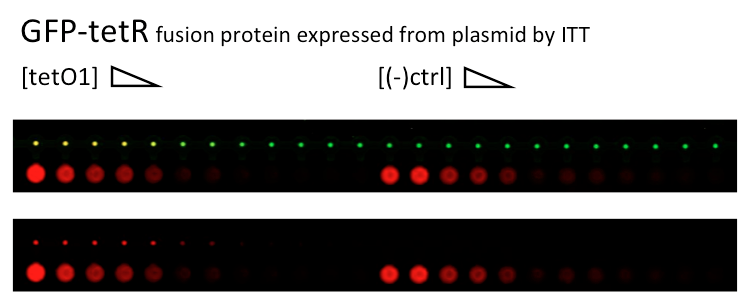
Thursday, 30 June 2011
Alina and Lilia did MITOMI on tetR-GFP expressed from plasmid, it was loaded on chip in ITT expression mix. DNA was spotted on June 29 in different concentrations for both: consensus (tetO1) sequence and a random sequence ((-)control).
Images: Green fluorescence comes from GFP-TetR and red fluorescence comes from DNA (cy-5 labeled).
This experiment confirms that wtTetR is expressed and correctly folded by SP6 expression system. The TF recognizes tetO1 consensus sequence while the tested random sequence (-)control has a significantly lower binding affinity to TetR.
Gibson Assembly
On the Gibson assembly side of things, Vincent and Irina purified the PCR product of both the J61002 plasmid and the pTet-RFP construct using the standard PCR purification protocol. Then, using NanoDrop, they measured the concentrations of J61002 plasmid and of the RFP construct:
RFP (820 bp) : 28.8 ng/microL
J6 (2334 bp) : 26 ng/microL
Instead of following the instructions on the wiki by Nikolaus Obholzer which has the wrong formula for finding the equal molecular ratio, we determined that the ratio ought to be 80/233 since the length of the RFP fragment is 820 bp and the length of the J6 plasmid is 2334. To make the Gibson mix, we used 2.6 microliters (80 ng % 28.8 ng/microL) of the RFP segment and 9 microliters of the plasmid (230 ng % 26 ng/microL). The assembly was done with a sample of 5 microliters taken from the 11.6 microliter mix.
We then did a transformation with these Gibson-ed plasmids and plated the cells on ampicillin plates and put them in the incubator overnight.
Gradient PCR
In a last attempt to try to amplify the P23019 plasmid backbone (the last piece needed to start a Gibson assembly of the TetR plasmid), Vincent tried a gradient PCR in which separate samples of the plasmid would be run at temperatures between 40 and 50 C (40, 42, 44, 46, and 48). The results showed, once again, that amplification was beyond reach.
 "
"