Team:Cornell/Description
From 2011.igem.org
(→Overall Project Description) |
(→Light Induced Lysis) |
||
| Line 13: | Line 13: | ||
=Light Induced Lysis= | =Light Induced Lysis= | ||
[[File:Cornell11_LightlysisDNA.png]] | [[File:Cornell11_LightlysisDNA.png]] | ||
| + | We would like to develop a genetic switch that is sensitive to specific wavelengths of visible light and use this gene expression system to lyse bacterial cells solely with light. The genetic light sensor is based on Chris Voigt’s “Multichromatic Control of Gene Expression in Escherichia coli,” which uses visible green light at 532 nm to induce specific gene expression. The system is composed of a light-activated surface protein, which autophosphorylates an intermediate chromophore, and a reporter protein that binds to a specific promoter and is activated by the chromophore. | ||
| + | |||
| + | The genes to be expressed downstream of the promoter make up a lysis cassette derived from the lambda phage and developed by Prof. Young at Texas A&M University. This lysis system is very useful because the incubation period after gene expression is on the order of 50 minutes, and the actual lysis occurs within a matter of one minute thereafter. Our hope is to lyse bacterial cultures within a known timeframe and with specificity to green light. | ||
=Violacein Pathway= | =Violacein Pathway= | ||
Revision as of 04:39, 28 September 2011
Project Description |
Future Directions |
Business Development |
Outreach/HP |
Safety
Contents |
Overall Project Description
Cornell’s BioFactory aims to develop a simple and efficient method for the construction of enzyme-immobilized surfaces capable of multi step chemical reactions.
As more chemical production techniques begin to utilize enzymatic reactions, genetic engineers must consider ways to resolve competing side reactions, the toxic accumulation of intermediates, reduce purification costs, and correctly expressing non-native enzymes or proteins in bacteria. In some cases, such challenges could be more easily resolved by simply extracting the molecular metabolic mechanisms and produce the target compound in a cell-free environment. We believe a cell-free system for biosynthesis can resolve these issues and still use the power of bacteria to help build devices capable of producing complex organic compounds.
Over the summer, Cornell ‘s iGEM team engineered strains of E. Coli to produce modified enzymes from the biosynthetic pathway of Violacein which were immobilized on the surface of microfluidic devices and capable of converting an initial feed of substrate into prodeoxyvioalcein, a direct intermediate of the violacein pathway. The microfluidic chips were designed, built and tested in the lab using Cornell’s modern cleanroom and nanofabrication facilities. We additionally design and began construction of a light-induced apoptosis system capable of lysing bacteria cultures producing the necessary enzymes without the use of expensive reagents or extensive protocols.
Light Induced Lysis
 We would like to develop a genetic switch that is sensitive to specific wavelengths of visible light and use this gene expression system to lyse bacterial cells solely with light. The genetic light sensor is based on Chris Voigt’s “Multichromatic Control of Gene Expression in Escherichia coli,” which uses visible green light at 532 nm to induce specific gene expression. The system is composed of a light-activated surface protein, which autophosphorylates an intermediate chromophore, and a reporter protein that binds to a specific promoter and is activated by the chromophore.
We would like to develop a genetic switch that is sensitive to specific wavelengths of visible light and use this gene expression system to lyse bacterial cells solely with light. The genetic light sensor is based on Chris Voigt’s “Multichromatic Control of Gene Expression in Escherichia coli,” which uses visible green light at 532 nm to induce specific gene expression. The system is composed of a light-activated surface protein, which autophosphorylates an intermediate chromophore, and a reporter protein that binds to a specific promoter and is activated by the chromophore.
The genes to be expressed downstream of the promoter make up a lysis cassette derived from the lambda phage and developed by Prof. Young at Texas A&M University. This lysis system is very useful because the incubation period after gene expression is on the order of 50 minutes, and the actual lysis occurs within a matter of one minute thereafter. Our hope is to lyse bacterial cultures within a known timeframe and with specificity to green light.
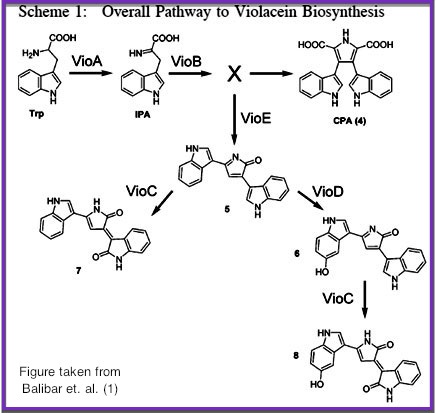
Violacein Pathway
We chose to use the violacein pathway fully characterized by Balibar et. al.¹ as our model enzyme-mediated-reaction. The violacein pathway involves five enzymes, VioA, VioB, VioC, VioD, and VioE, in the conversion of L-Tryptophan into a violacein, purple chromophore. This pathway was an especially attractive candidate for our project not only because it has been thoroughly characterized in E coli., but also because relatively few enzymes are needed to convert a cheap, common substrate into a visualizable. Furthermore, only three of the five enzymes (VioA, VioB, and VioE) are required to produce a colored produce. Even with the exception of Vios C and D, L-Tryptophan is converted into prodeoxyviolacein, a green pigment (Figure 3A in Balibar et. al.¹). Thus, we chose to biotinylate only VioA, VioB, and VioE to provide proof-of-concept that enzymes binded to our microfluidic devices may be used to facilitate enzyme-mediated reactions.
Violacein Pathway
Microfluidic Device
DNA Assembly Methods
1. In Vitro Biosynthesis of Violacein from l-Tryptophan by the Enzymes VioA−E from Chromobacterium violaceum†
Carl J. Balibar and and Christopher T. Walsh Biochemistry 2006 45 (51), 15444-15457. http://pubs.acs.org/doi/abs/10.1021/bi061998z "
"