Team:CongoDRC-Bel Campus/Project
From 2011.igem.org
| You can write a background of your team here. Give us a background of your team, the members, etc. Or tell us more about something of your choosing. | |
|
Tell us more about your project. Give us background. Use this is the abstract of your project. Be descriptive but concise (1-2 paragraphs) | |
| Team Example |
| Home | Team | Official Team Profile | Project | Parts Submitted to the Registry | Modeling | Notebook | Safety | Attributions |
|---|
ABSRTACT
NB. We have two differents approaches for this project: modelling transmission and engineered mycobacterial vaccine.
1.TRANSMISSION
Buruli Ulcer (BU) is a debilitating disease that mainly affects the skin but which can also affect the bone. The causative agent is called Mycobacterium ulcerans, which although different, belongs to the same family of organisms that cause leprosy and tuberculosis. This affection is one of the most common mycobacterial diseases in human health. Several cases of BU have been identified in at least 26 countries in the African Region since the early 1940s. However, in recent years, an increasing number of cases have been recorded in nearly all Western Africa countries, along the Gulf of Guinea. The causative agent is Mycobacterium ulcerans. For it to cause disease, it must either be transmitted from one infected individual directly to another (e.g.; direct contact) or through the environment (e.g.; via an intermediate host) to a susceptible host. It must also have the capability of surviving and multiplying in either the environment or the host or in both.
Generally, the reservoir of infection is not exactly known but is believed to be either the sick individual or the environment, especially marshy soil and vegetation along slow-flowing streams and rivers, or perhaps an intermediate host that thrives in such environment conditions. The latent period for infection to progress to disease in the susceptible host is also not well known. The mode of transmission is not entirely known. Recent evidence suggests that certain aquatic insects (aquatic bug) belonging to the genus Naucoris and Diplonychus may be involved in the transmission of the infection. The causative agent is commonly introduced into the skin (from surface contamination) through traumatic breaches in the skin (irrespective of size). However, it is not well understood whether infection can occur directly through the intact skin, after an insect bite, or by direct person –to-person contact.
One interesting hypothesis concerns the possibility of transmission by some aquatic species. Nevertheless, this bacteria it’s environmental, the simple hypothesis is the direct transmission of M.ulcerans by contaminated water. To identify the more probable way of transmission of M.ulcerans in environment, we rely on data and mathematical modeling transmission of M.ulcerans. Using Synthetic biology approaches we managed to build two different epidemiological models taken account of these two ways of possible transmission. The first model concerns the “environmental” transmission by contact with mycobacteria, through contaminated water. The second epidemiological model concerns the transmission of mycobacterium by ecological “networks”. The parameters of these two epidemiological models will be estimated from data of field with the more probable mode of transmission.
2.VACCINE.
M. ulcerans causing by B.U. This infection can be treated with multi drugs regime (rifampicin and streptomycin) but this is often associated with induced antibiotic resistance and does not protect individuals from re-infection Vaccination against M. ulcerans can therefore be aviable alternator to control this wide spread infection. However, developing an effective vaccine against M. ulcerans has presented a challenge because M. ulcérans or its components, which have frequently been used as parts of vaccines, are modified by mycobacterium such that they avoid host defense mechanism using synthetic biology approaches, we managed to assemble functional ‘’immunobrick’’ into a designer vaccine with a goal to activate both innate and acquired immune response to M. ulcerans. We propose developing two forms of such designers’ vaccines. -One will be based on modifying M. ulcerans component such that it can now recognize by the immune system. -The other relied upon linking M. ulcerans component to certain molecule of the innate immune response (so called Toll like receptor) to activate and guide M. ulcerans proteins to relevant compartments within the immune cell causing optimal innate and acquired immune response. An effective vaccine against M. ulcérans is not available, although it will be a durable solution. M. ulcerans avoids the immune surveillance by modifying several of this components including LAM (lipoarabinoman) ,SL1(sulfolipide 1) mycolactone to avoid detection by several Toll Like Receptor. The goal of our project was to prepare a modular designer vaccine using the principal of synthetic immunology. An effective vaccine was to trigger activation of adaptive immunity, against microbial proteins (mycolactone), polysaccharides or lipids metabolites (PDM: phtiocerol dimycocerosate, PGL: phenolglycolipide, SL1: sulfolipide 1, GPL: glycopeptidolipide) as well as innate immunity, with which is usually achieved by addition of adjuvant of whole microbes. We prepared a set of ‘immunobricks’ with the defined functions in activation of the immunosystem and can be combined to active a desired response. In the first approach, we want to modify the components M.ulcerans wall (LAM, SL1, PGL, PDM, and GPL) to be able to active TLR 2 making it visible to the immune system.
Contents |
Project Details
WHY THIS CHOICE? BURULI ULCER
1. INTRODUCTION:
Buruli Ulcer (BU) is a debilitating disease that mainly affects the skin but which can also affect the bone. The causative agent is called Mycobacterium ulcerans, which although different, belongs to the same family of organisms that cause leprosy and tuberculosis. This affection is one of the most common mycobacterial diseases in human health. Several cases of BU have been identified in at least 26 countries in the African Region since the early 1940s. However, in recent years, an increasing number of cases have been recorded in nearly all Western Africa countries, along the Gulf of Guinea. The disease is currently known to be endemic there in the following countries: Benin, Ivory Coast, Ghana, Guinea, Liberia, Nigeria, Sierra Leone and Togo (see Africa map).
2. HISTORICAL OVERVIEW: In 1897, Sir Robert Cook described large ulcers in Uganda, which were almost certainly caused by M. ulcerans. It was, however, not until 1940 in Bairnsdale, Australia, when Mac Cullum, first described the causative agent of this disease, after discovering the acid-fast-bacill (AFB) in a biopsy specimen from a leg ulcer in a young child. In 1948, he published the first clinical description of this new mycobacterial infection. Between 1923 and 1935, a missionary physician working in the North-eastern area of Democratic Republic of Congo (Zaire) observed undermined skin ulcers that were rich in AFB.
3. THE CAUSETIVE AGENTS:
The causative agent is Mycobacterium ulcerans. For it to cause disease, it must either be transmitted from one infected individual directly to another (e.g.; direct contact) or through the environment (e.g.; via an intermediate host) to a susceptible host. It must also have the capability of surviving and multiplying in either the environment or the host or in both.
Generally, the reservoir of infection is not exactly known but is believed to be either the sick individual or the environment, especially marshy soil and vegetation along slow-flowing streams and rivers, or perhaps an intermediate host that thrives in such environment conditions. The latent period for infection to progress to disease in the susceptible host is also not well known.
 [[File:Shematic cut of aquatic bug Naucoris cimicoides
.jpg]]
[[File:Shematic cut of aquatic bug Naucoris cimicoides
.jpg]]
4. MODE OF TRANSMISSION The mode of transmission is not entirely known. Recent evidence suggests that certain aquatic insects (aquatic bug) belonging to the genus Naucoris and Diplonychus may be involved in the transmission of the infection. The causative agent is commonly introduced into the skin (from surface contamination) through traumatic breaches in the skin (irrespective of size). However, it is not well understood whether infection can occur directly through the intact skin, after an insect bite, or by direct person –to-person contact. After entering the subcutaneous tissue of the host, Mycobacterium ulcerans is known to produce a toxin that causes its characteristic pathology from nodules, edematous lesions to skin ulcerations.
5. EPIDEMIOLOGY:
To better design a public health intervention to control this disease in Africa, it is imperative to understand its epidemiology. However, this subject is still under intense investigation in several countries in the region.
5.1. IN THE WORLD:
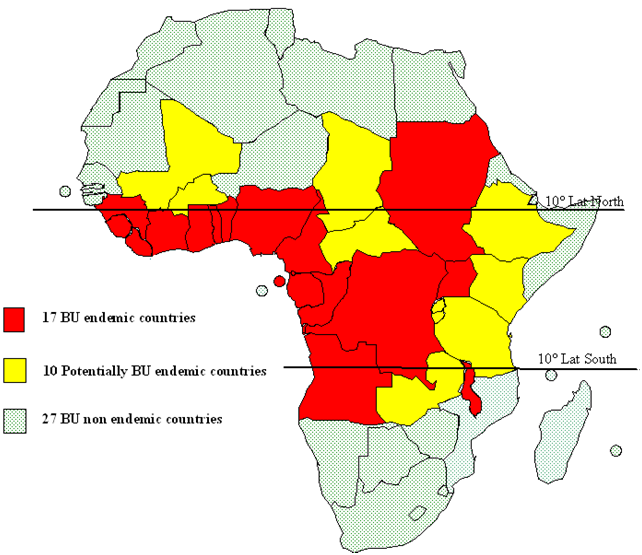
Buruli Ulcer is notified in almost 30 countries with tropical and subtropical climates, including in America, Asia, Oceania and Africa. But the disease may also occur in some countries where it has not yet been recognized.
The map below represents almost the countries of each continent:
5.2. GEOGRAPHIC DISTRIBUTION IN AFRICAN REGION:
Nevertheless, it is important to briefly describe the current state of knowledge about the subject in order to understand the challenges that confront us in combating this mysterious disease.
The current geographic distribution of the disease in African Region is depicted in Fig.2. To date, 21 Member States (old and new foci) in Western (9 countries), Central (6 countries), Eastern (4 countries) and Southern Africa (2 countries) have reported cases (both suspected and confirmed). Countries in Westen Africa, however, have reported the highest number of cases (cumulative range of 1,000 to 10,000), with an endemic pattern in recent years.
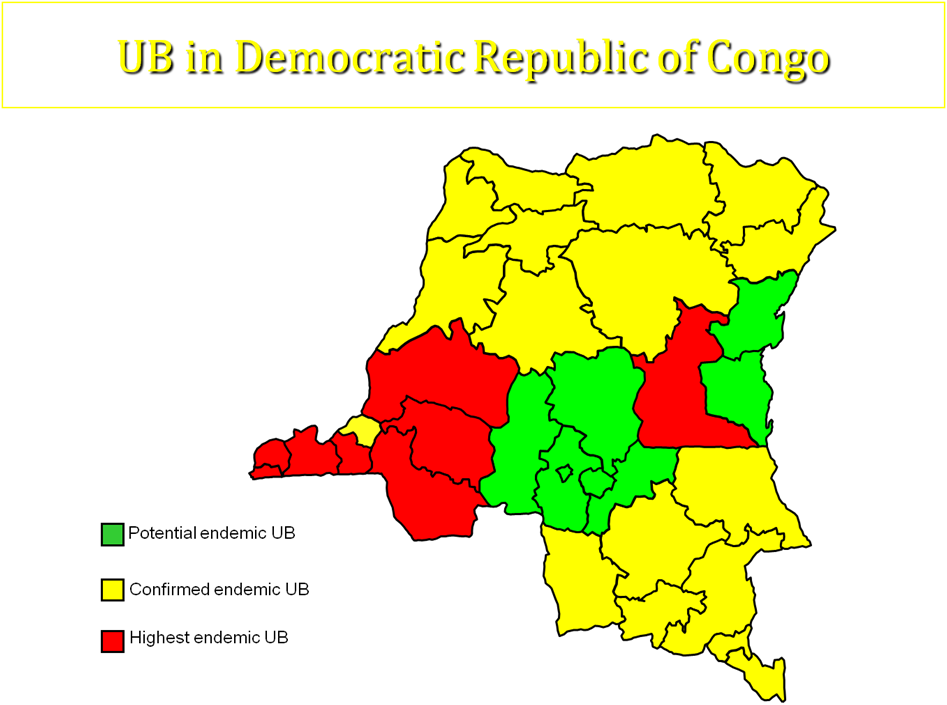
5.3. GEOGRAPHIC DISTRIBUTION IN DEMOCRATIC REPUBLIC OF CONGO REGION
The first case of UB in DRC has been reported in 1950. In 2004, the national investigation has shown that there were some suspected cases of UB in 6 of 11 regions. Three(3) of these are considerated highest endemic: Bas congo, Bandundu and Maniema; whereas others (3) are less endemic ( Equateur, Katanga and Province orientale).
File:BU in DRC in 2004 and 2008.jpg
5.3.1. AGE AND SEX DISTRIBUTION:
In general, the disease can affect all age groups. However, children under 15 years of age (range of 2-14 years) are predominantly affected. There are no gender defenses in the distribution of cases among children, although some studies have reported more females than males among affected adults. This observation is believed to be caused by differential gender exposure to the organism in environment rather than differential susceptibility to infection.

5.3.2. DISTRIBUTION OF SITE LESIONS:
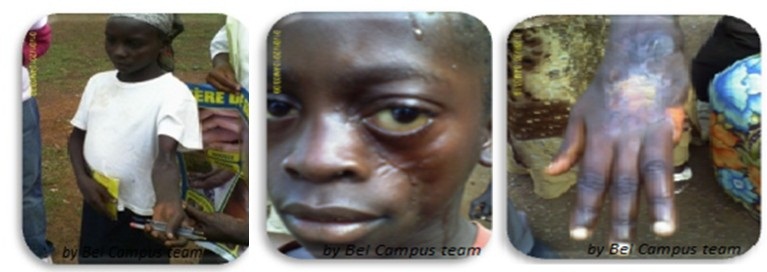
Most ulcers occur on the extremities, as they are generally more exposed and prone to injury. Lesions on the lower extremities are almost twice as common as those on the upper extremities. Ulcers on the head and trunk are less common. Recurrence of disease at the same or other sites is common, implying that immunity to infection is generally minimal.
[[File:Eight year old girl with her right limbs strongly affected
.jpg]]
5.3.3. MAGNITUDE OF DISEASE Until recently, in several countries where the disease has been reported, it is not considered to be a public health problem, hence the exact distribution and number of cases are not known. Available data, mostly obtained through passive case finding in a few communities where the prevalence has been estimated, show fairly high prevalence rates.
5.3.4. ENVIRONMENTAL FACTORS
BU generally occurs in warm, humid environments (tropical and subtropical regions) especially in areas with marshy soil and stagnant or slow flowing water bodies. Man made topographical modifications such as damming of rivers and stream, mining activities and deforestation are believed to create favorable environmental conditions for the survival of the organism and the disease it causes. The occurrence of the disease has also been observed to follow some seasonal pattern, generally increasing during the rainy season and coinciding with periods of farming activities.

6. PATHOLOGY
6.1. ACTIVE DISEASE
The term “active disease” generally refers to ongoing infections.
During the pre-ulcerative stage the disease, the following forms are the clinically recognizable:
Papule: usually painless, sometimes itches, not tender, palpable, intra-dermal lesion
Nodule: usually painless, palpable, firm lesion, 1-2 cm in diameter situated in the subcutaneous tissue and typically attached to the skin. The skin around the lesion is usually hypo-pigmented in dark skinned people.

Plaque: usually painless, well delimited, elevated and firm lesion, more than 2 cm in diameter Edema: diffuse, extensive non-pitting, swelling, ill-defined margins, firm, usually painful with or without color change others affected skin. Ulcerated Stage: A typical Buruli Ulcer is defined as a skin ulcer characterized by necrotic center, undermined edges and edematous skin. In the absence of the secondary bacterial infection, the ulcer is usually painless or minimally painful ; two mains clinical entities at this stage of disease may be differentiated as follows:
Small ulcer: an ulcer with a size less than 2 cm in diameter.

Large ulcer : an ulcer with a size greater than 2 cm in diameter
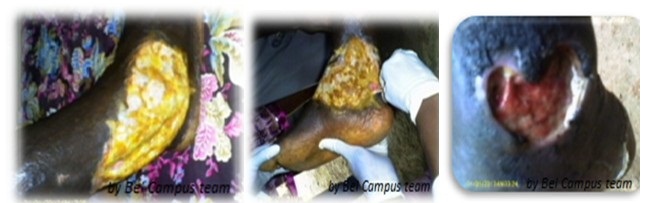
6.2. INACTIVE DISEASE
This refers to healed lesions that have a characteristic, depressed, star-shaped scar. There are three mains clinical entities:
• Scar without sequel
• Scar with sequel ( e.g. contracture deformities, amputation, loss of organs)
• A mixture of the above
 "
"



