August 22
Send BPA and degraded products to HPLC for analysis
Send 16s for sequencing using FWD and REV.
PCR'd R0040, K123001, B0014 and pSB3K3 for Gibson assembly of pNT002
Results
Plated 16s and WT-F87A cultures show many colonies
Mix 10% glycerol solution and place in 4°C fridge for tomorrow's electrocompetence method
Overnight shake culture of MG-1655 experimental E. coli strain
August 23
Make experimental MG-1655 strain electrocompetent
Made 53 plates today!
- 24 LB-amp plates
- 16 LB-chlor plates
- 13 LB plates
PCR'd R0040 and pSB3K3 for Gibson assembly of pNT002
PCR'd the DDT gene with new primers to add stop codons and biobrick ends.
PCR'd the DDT gene with BamHI and XbaI sites and a C-terminus his tag for insertion into pET11-a. This will allow us to overexpress the protein for purification and characterization.
PCR'd WT-F87A with biobrick ends.
Dpn 1 digested the DDT and p450 PCRs overnight
August 24
Visited the San Jose Creek Water Reclamation Plant
Made more Gibson mix according to Joe's protocol.
Did double restriction digest of DDT gene and p450 PCRs with biobrick ends and pSB1C3 using EcoRI and PstI.
Did double restriction digest of DDT gene PCR with pET11-a insertion endings and pET11-a using BamHI and XbaI
Did CIP digest of pET11a and pSB1C3
Ligated DDT gene with biobrick ends into pSB1C3
Ligated p450 with biobrick ends into pSB1C3
Ligated DDT gene into pET11-a
Transformed all ligations into 50 ul aliquots of DH5a electrocompetent cells
Transformed 1 ul of 10 pg/ul pUC19 into 1 aliquot of DH5a electrocompetent cells
Started overnight of pSB1C3 so there is more DNA available to use as backbones for biobrick parts to send to the registry.
X-gal plating shows blue colonies; overnights of J23119-MG1655 and R0010-MG1655 to make glycerol stocks
August 25
pSB1C3 concentration 118.0 ng/ul
Calculated transformation efficiency of DH5a electrocompetent cells is about 2x10^8 cfu/ug
Results
| Name |
Number of colonies |
| p450 biobricked in pSB1C3 |
~3400 |
| DDT gene biobrick now with stop codons in pSB1C3 |
~6400 |
| pSB1C3 ligation negative control |
0 |
| DDT gene inserted into pET11-a |
~6400 |
| pET11-a ligation negative control |
27 |
| pUC 19 in DH5a electrocompetent cells |
976 |
| DDT gene PIPE |
0 |
| VPIPE negative control |
0 |
| pNT002 + in commercial XL-10 gold |
54 |
| pNT002 - in commercial XL-10 gold |
28 |
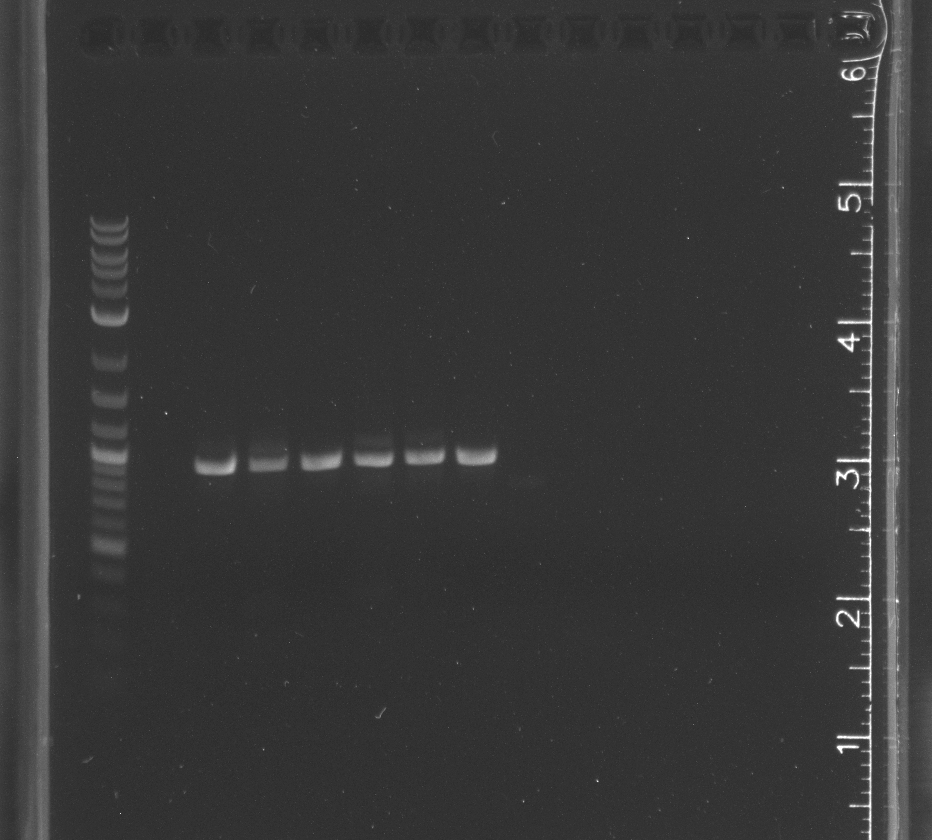
CPCR of DDT gene lane 1 NEB 2-log ladder, 3-8 with in biocrick form, 9-14 in pET11a
|
CPCR of WT-F78A in biobrick form, lane 1 NEB 2-log ladder, 3-8 WT-F87A
|
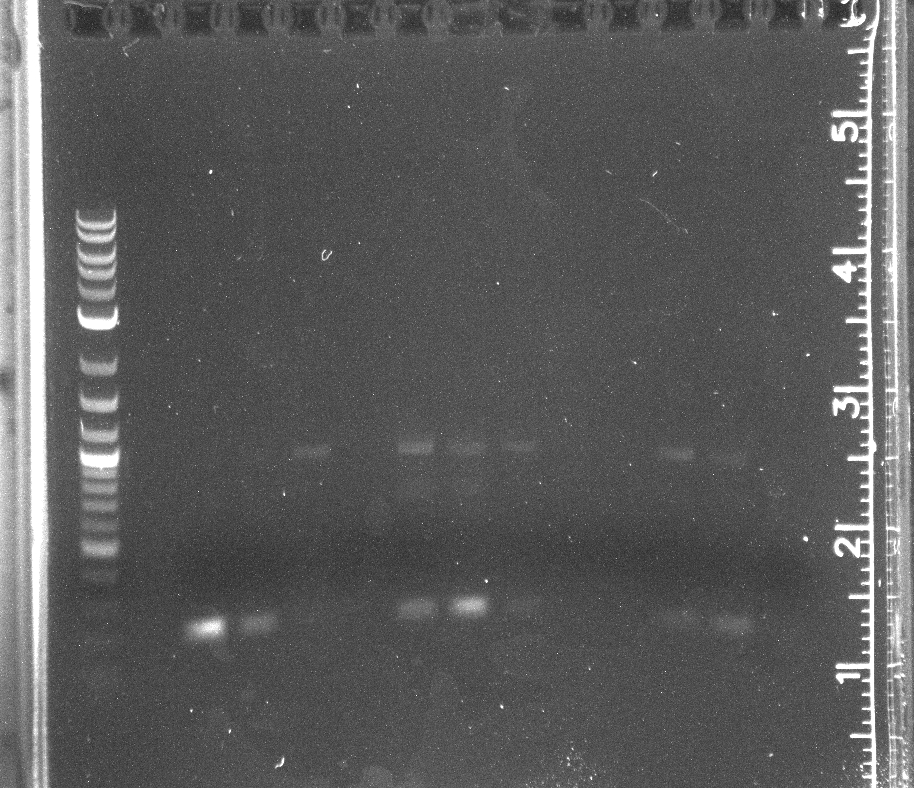
CPCR of pNT002 constructed by Gibson, lane 1 NEB-2 log ladder, 3-14 pNT002
|
August 26
Sent improved DDT biobrick and DDT in pET11a off fro sequencing
Added X-gal to J23119 overnights to test for expression
Results
J23119-MG1655 culture turned X-gal blue; use spectrophotometer for better measurement
Started culture of J23119-MG1655 in flask with glass beads for biofilm/X-gal experiment
August 27
Moved from Braun 16 to Keck 040
 "
"