Team:Caltech/Project
From 2011.igem.org
|
Project |
Bioremediation of Endocrine Disruptors Using Genetically Modified Escherichia ColiFile:Caltech team.png Your team picture Endocrine disruptors, or substances that mimic estrogen in the body, have detrimental biological effects on the reproduction of several species of fish and birds; the Caltech team focuses on bioremediation of these toxins. Our goal is to create a system housed in E. coli that can be used to process water and remove endocrine disruptors on a large scale. We focus on isolating degradation systems for the common endocrine disruptors bisphenol A (BPA), DDT, nonylphenol and 17a-ethynylestradiol. We synthesized known degradation enzymes DDT dehydrochlorinase, BisdA and BisdB, and characterized the behavior of these enzymes when acting on our target endocrine disruptors. In addition, we explored the potential of certain cytochrome p450s to initiate degradation of these chemicals, focusing on WT-F87A degradation of BPA. Finally, we characterized the functionality of E. coli protein processing when E. coli is deployed as an easily containable biofilm on various substances in aqueous environments. Introduction:Endocrine-disrupting chemicals (EDCs) are chemicals that interact with the endocrine system by binding to hormone receptors, causing problems in sexual development and reproduction of organisms. These chemicals are introduced to the environment from improper disposal of plastic wastes, hormonal medications remaining in human waste, and pesticides. Areas with high concentrations of estrogen in water have been shown to correlate with a higher percentage of intersex fish, and pesticides such as DDT have been shown to impact the development of the female reproductive tract in birds. Many EDCs are persistent organic pollutants, and even though regulations have been put in place for pesticide use and industrial production of endocrine disruptors, many of these chemicals continue to pollute bodies of water in significant concentrations. BisdA and BisdBWe sourced genetic material for BisdA and BisdB from the Registry of Standard Biological Parts. The sequence listed for these parts had the wrong codons, so we sequenced the parts to determine the correct codons. We then designed genetic constructs with inducible promoters, ribosome binding regions, and terminators in plasmid backbones for each gene. We accessed these parts by transforming DNA from the Registry of Standard Biological Parts into chemically competent E. coli for construction, and used PCR to extract our desired components. We attempted several methods of assembly for these pieces, including traditional assembly, Gibson assembly, and a combination of PCR assembly for the coding construct and standard assembly to insert the coding construct into a backbone. After assembly of these components was complete, we combined the two coding constructs into one vector and expressed this vector in E. coli for experimentation. We are now inducing production of BisdA and BisdB so that we can investigate their ability to degrade EDCs. Gibson AssemblyWe attempted to use Gibson Assembly for creating composite BioBrick plasmids including inducible BisdA and BisdB. After many weeks of doing Gibson reactions and screening for colonies, we have observed that Gibson assembly may not be the most efficient and reliable method of assembling BioBricks. Gibson assembly may be a quicker method of cloning than using restriction enzymes and ligase, but this work indicates that quality and quantity of plasmids produced using this method are not as easily reproducible as standard assembly for assembly of multiple BioBrick parts is. If iGEM teams wish to use this method for assembly, we have shown that limiting the total DNA in the Gibson reaction, limiting the number of parts being combined in the reaction and using extremely competent cells improves the ratio of experimental colonies to negative control colonies. These can then be screened using colony PCR or sequencing. However, the high complementarity between the BioBrick prefix and suffix could contribute to the high numbers of self-ligation we observed. Due to the enzymes in the Gibson reaction, phosphatase cannot be used, a common procedure in standard assembly. DDT Dehydrochlorinase
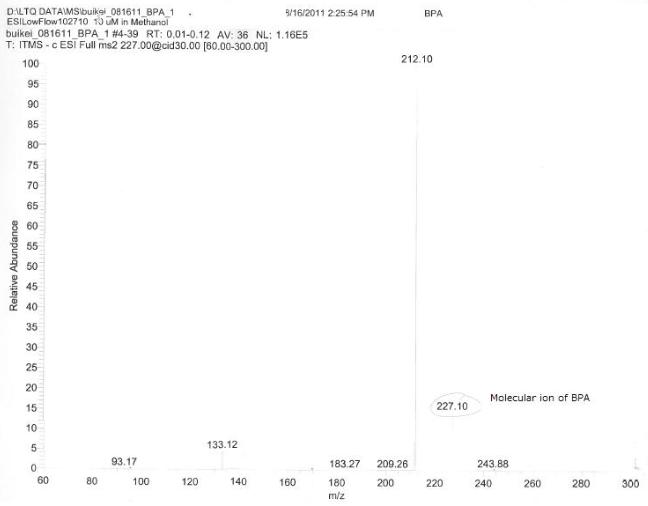
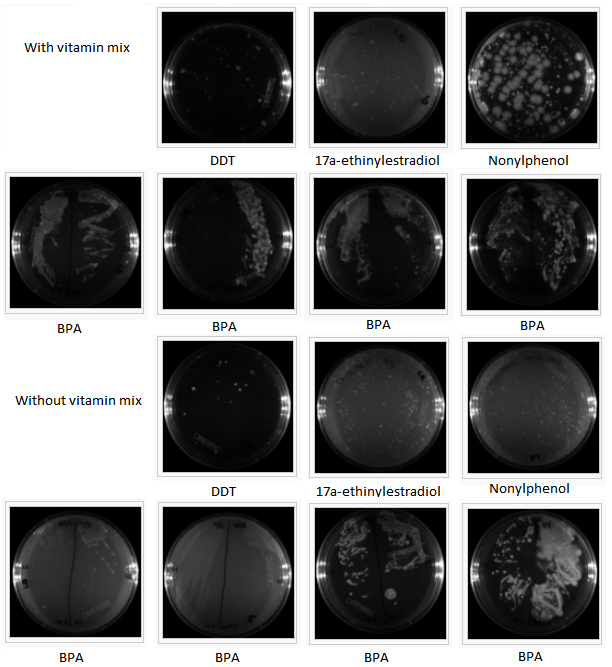
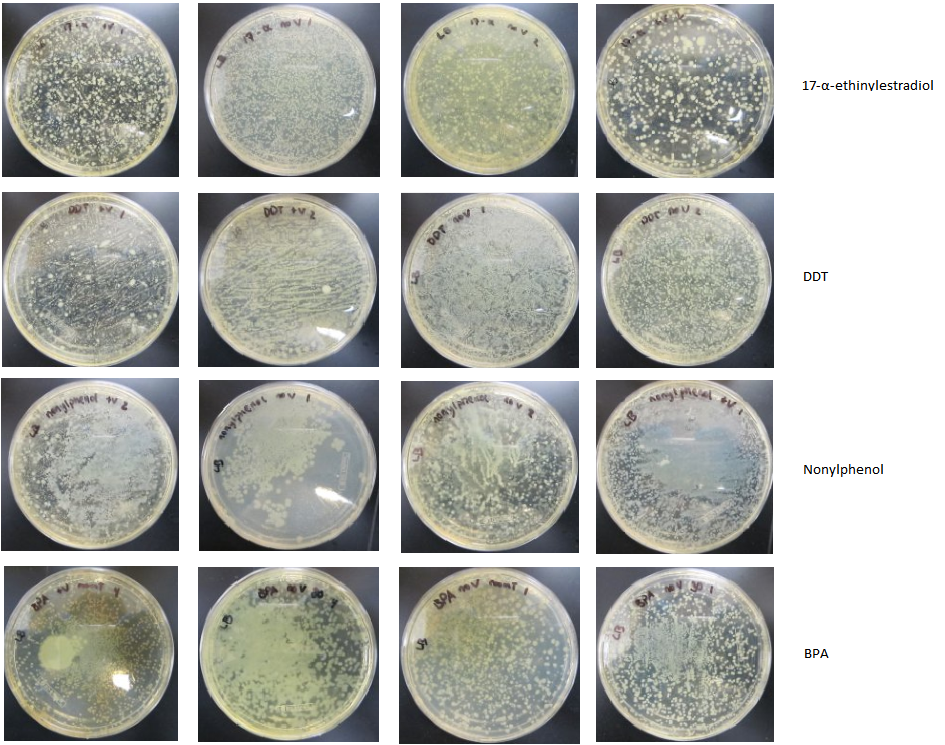
Cytochrome p450sCytochrome p450s are known to be initiators of degradation for several compounds. We analyzed the structures of the cytochrome p450s which the Arnold Lab possessed in their genetic library and selected four highly promiscuous p450s. We then conducted reactions of these p450s with BPA, DDT, 17a-ethinylestradiol, and nonylphenol. We attempted to analyze the product of these reactions with HPLC and with GCMS, but we found that the product did not stay ionized and therefore could not be analyzed. We formed an electrospray of the reaction products and analyzed this using HPLC, and we were able to see degradation of BPA into a different compound in the results. Selection of EDC-Degrading OrganismsIn order to identify an organism capable of degrading our EDCs, we collected dry and wet samples from the Los Angeles river and used these samples to inoculate liquid minimal media cultures in which the sole source of carbon was the EDC. (We used media with and without a vitamin mix to account for any compounds the organisms might not be capable of manufacturing themselves, but the presence or absence of the vitamin mix seems to have made little difference in the growth of the organisms; cultures exhibited equivalent growth both with and without the vitamin mix.) We sequentially used these cultures to inoculate new cultures every two to three days over an eight-week period in order to further isolate organisms. At the third week we plated the cultures on LB plates and observed significant growth of many different types of organisms (figure 1), indicating that our cultures contained organisms capable of surviving on our EDCs. To further isolate these organisms, colonies from each culture were resuspended in liquid minimal media; as before, new liquid minimal media cultures were inoculated from each of these every two to three days. The cultures were plated again on LB in the eighth week (figure 2) and again, significant growth was observed. At this stage, we attempted to plate our cultures on solid minimal media; however, all of the compounds we chose are insoluble in water, which makes traditional plating impossible. We attempted several alternate methods of plating the EDCs together with the minimal media and cultures, but all these attempts were unsuccessful. Thus, although we demonstrated the presence of EDC-degrading organisms in our cultures, we were unable to fully isolate any organisms for further study.
Biofilm ColumnsTo prepare biofilms, we first tested the length of time it takes for a biofilm to form in a 96 well plate using crystal violet.
|
 "
"