Team:Brown-Stanford/FRETSensor/Construct
From 2011.igem.org
Our Construct
Requirements
During our brainstorming sessions, we identified several requirements for our FRET-based biosensor:
- It must be easily modified to report on any number of environmental or intracellular factors
- The signal must be specific for only a single stimulant
- The transcriptional element must be as small as possible to minimize time between detection and reporting
- The initial biosensor will detect DNA damage caused by UV radiation
Construct Design
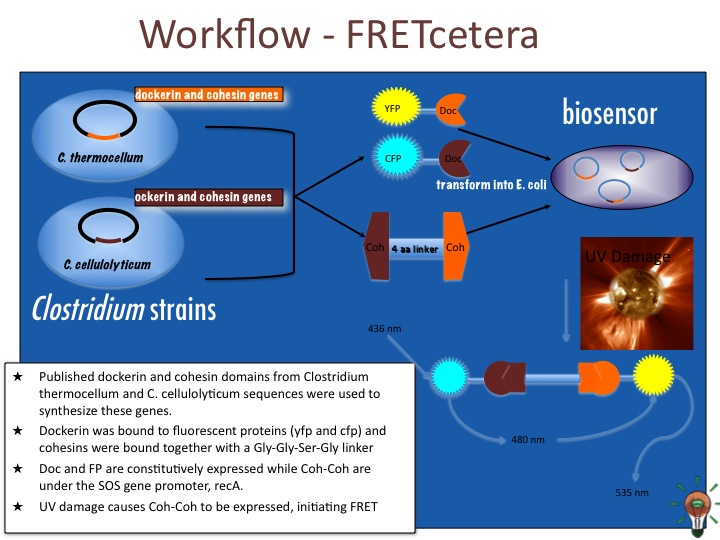
Our construct involves constitutive expression of CFP and YFP. The CFP is bound to a dockerin domain of Clostridium thermocellum with a scaffoldin linker and the YFP bound to a dockerin domain of Clostridium cellulolyticum. To bring these two together, an inducible promoter will produce a cohesin-cohesin fusion protein. This construct will consist consist of a C. thermocellum cohesin bound by Gly-Ser-Gly to a C. cellulolyticum cohesin. Because cohesin-dockerin interactions are species specific, the cohesin fusion protein will bring together exactly one each of CFP and YFP to within 10 nm in order to generate a FRET signal.
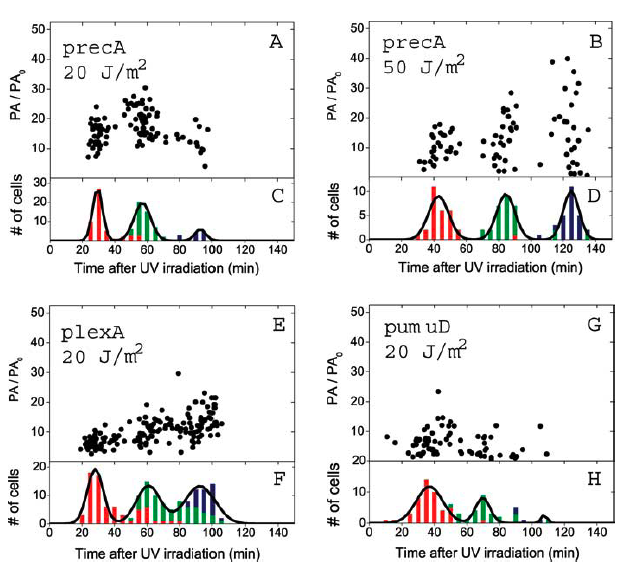
The promotor for this particular construct is IPTG inducible to demonstrate the principle of FRET. During DNA damage, however, bacteria activate what is known as SOS genes as a way of mediating and repairing their genetic material. These SOS genes include recA, lexA, and umuDC, among others. In particular, umuDC is responsible for regulating discrete peaks of SOS promoter activity following radiation of over 10 J/m2. 1
In our proposed design, the cell will constitutively express CFP and YFP bound to dockerin domains at the C or N terminus (depending on which does not interfere with protein folding and also does not obscure the doc domain). Upon encountering a UV radiation dose, the promoter for umuDC upstream of our third construct will begin transcription of the cohesin fusion protein. The cohesin fusion protein will bind the CFP and YFP, generating a FRET signal, which will be read automatically by a digital sensor.
Current and Future Status of FRET
We synthesized each of our constructs from [http://www.dna20.com DNA 2.0] and [http://www.invitrogen.com Invitrogen]. However, because the constructs did not arrive until midway through August, we were not able to fully characterize or troubleshoot them. They are currently residing in our -86°C freezer and we will continue testing them for functionality.
In a Martian colony that heavily relies on transgenic microorganisms for its functioning, it is necessary to have a system of monitoring the health of each culture. In a fermentation culture, plasmid loss is a function of time, cell density, and declines by the hour. In high cell density bioreactors, problems most often arise with scale-up issues, plasmid instability, and substrate inhibition.2 In order to make a robust bioreactor on Mars, one must begin with hardy organisms. That is why we hope to see in the future more attempts to solving scale-up problems such as including a “[http://www.pnas.org/content/early/2010/08/09/1009747107.abstract kill-switch]” for under-performing bacteria, [http://jb.asm.org/cgi/content/abstract/191/16/5240 directed evolution] for UV resistance, and efforts to reduce [http://www.ncbi.nlm.nih.gov/pubmed/10797246 contamination]. On Mars, there is also the problem of UV damage, especially for those culture that a meant to work with minimal shielding, such as the PowerCell cyanobacterial cultures. Although our system may not solve some of the scale-up issues on Earth, self-reporting cells would be a step towards quick detection of problems in a Mars bioreactor.
The FRET mechanism has many uses besides DNA damage detection. For example, due to the number of different combinations of FRET chromophore pairs, a diversity of responses can be engineered into different reporting strains of bacteria to detect any signal that is mediated by a promotor.
References
1 Friedman, Nir et al. (2005) Precise temporal modulation in the response of the SOS DNA repair network in individual bacteria. PLOS Biology 3.7, 1261-1268.
2 Tripathi, NK et al. (2009) High Yield Production of Heterologous Proteins with Escherichia coli. Defence of Science Journal 59.2, 137-146.
 "
"