|
|

Wednesday, June 1
- Made agar with 3.7 g / 100 ml DI water
- Made 2 plates from 2 MG1655 strains received yesterday
- Submitted synthesis requests
Thursday, June 2
- meeting with life sciences people explaining project
- single sided synthesis with phosphotase direction?
-
- 5 g peptone
- 2.5 g yeast extract
- 5 g NaCl
- 500 ml water
Friday, June 3
- got our synthesis order in
- did a 2nd large materials order
- met with barrett funding person
- meeting with Jon, grad student from Misera's lab
- lab:
- resuspended part E0840 from well following parts registry protocol
- followed "competent cells and chemical transformation procedure for DH5 alpha":
- made 20mM concentration mgcl2 in spun cells from yesterday
- spun for 2 hours in 37
Saturday, June 4
- made 500 ml SOC following protocol
- transformed resuspended dna into e coli
- followed transformation protocol, but did not use water bath
Monday, June 6
- transformed cells from saturday not growing
- test if competency procedure killed cells:
-
- repeat transformation with water bath instead of direct heat:
- thaw competent cells on ice
- 50 ul cells + 1 ul resuspended DNA, on ice for 30 minutes
- heat shock at 42 in a water bath for 60 seconds
- incubate on ice, 5 min
- add 100 ul SOC to cells
- shake at 37 C for 2 hours (11 am - 1 pm)
- plate 20 ul, 200 ul (2 plates)
- incubate overnight
Tuesday, June 7
- amp plates did not grow
- competent cells plated without amp grew
- new transformation using 2 different parts (did not work):
-
- control onto non amp plate to test if transformation killed cells (it did not)
Wednesday, June 8
- autoclaved everything
- made 200 ml new SOB, 50 ml SOC
- new transformation:
- top10 chemically competent e coli from biodesign
- part: BBa_E0840
- using top10 protocol
- plates: # 4 50ul, 5 150ul
- MG1655 plated from plate # 2:
- plate: # 1
- from: 6-2 plate MG1655
-
Thursday, June 9
- Xiao introduced 3 grad students who can offer advice/assistance throughout the project
- We will meet with them (likely Thursdays @ 10am) to update them on our progress
- #4, 5 have colonies but no glow with UV- no promoter in biobrick part
- need to add in a promoter
-
-
- use Knight restriction protocol
- cut promoter BBa_J23101 with ECORI, SPEI
- cut GFP generator BBa_e0840 with ECORI, XHOI
- DNA extraction- use "ethanol precipitation of nucleic acid" procedure
- Ligate
- Transform that
- Create stock of competent cells
- Order Top10 cells (what strain are these?)
- Make glycerol stock of biobrick
- Jon/Misra procedure for competent cells and transformation:
- overnight culture from previous:
- diluted 1 to 50
- shook 1 hr in 37
Friday, June 10
- 2 competency procedures (Jon, CCMB80)
- 3 transformation procedures (jon, CCMB80, top10 from biodesign)
- 12 plates made (see lab notebook)
- Autoclaved glass test tubes
- Made glycerol stock (ask Dan about procedure)
- Bought competent cells
-
- Got account set up (still need to create Sunrise account)
-
- Met w/ James Alling, JD-PhD interested in helping us out
- He is very good at speaking and could help w/presentation later
- Very attracted to promoting big picture of project
- Kylie determined new primers after noticing that we don't need Cas 1,2,3 for our natural cas construct (from "structural basis for CRISPR")
- This brings down total to 3.8kb instead of over 5kb
- We will try both ways, see if cas 1,2 do anything interesting
- primers for every CAS gene?
- Biobasic is taking twice as long as they advertised (no DNA until june 20?)
- From now on we will go through IDT
- Have contacted them about discount, will see what they say
Saturday, June 11
HAPPY 22ND BIRTHDAY KEITH!
Tuesday, June 14 - Friday, June 17
- Synthetic Biology 5.0 conference
Monday, June 20
- overnight culture x 2 (LB) for DNA
- overnight culture x 2 (LB, SOC) for culture
-
- genome prep (k12)
- pcr
- competent cells
Tuesday, June 21
-
- ruben, ethan
- made 250 ml SOB
- 9 210ul tubes, -80
- plates made:
- 1. LB, transformation: DA
- 2. LB + amp, transformation: DA
- 3. LB + amp, transformation: PUC19
- TSS procedure w/ BL21 cells
- madeline, juan, keith
- plates made:
- 4. LB + amp, PUC19, burned
- 5. LB + amp, PUC19, unburned
- 6. LB + amp, DA
- 7. LB + amp, DA
- 8. LB + +amp, DA
-
- 9. LB + amp, PU19
- 10. LB + amp, PUC19
- 11. LB + amp, DA
-
- First PCR overnight of CAS genes
Wednesday, June 22
- made new LB + amp stock
- made 200 ml LB + amp broth
- plates from yesterday:
- don't know if amp was any good
- 1: normal growth (no distinct colonies)
- 2: colonies
- 3: no growth
- 4: no growth
- 5: no growth
- 6: colonies
- 7: colonies
- 8: colonies
- 9: very heavy colonies
- 10: very heavy colonies
- 11: light colonies
- overnight cultures of 2, 6, 7, 8, 11 (3 each in LB + amp broth)
- gel of pcr product
- new plates:
- 1. CCMB80, LB, PUC19
- 2. CCMB80, LB, DB
- 3. CCMB80, LB, SEQ1
- 4. CCMB80, LB + amp, no plasmid
- 5. CCMB80, LB + amp, PUC19
- 6. CCMB80, LB + amp, DB
- 7. CCMB80, LB + amp, SEQ1
- 8. NEB, LB, PUC19
- 9. NEB, LB, DB
- 10. NEB, LB, SEQ1
- 11. NEB, LB, no plasmid
- 12. NEB, LB + amp, no plasmid
- 13. NEB, LB + amp, PUC19
- 14. NEB, LB + amp, DB
- 15. NEB, LB + amp, SEQ1
- 16. TSS, LB, PUC19
- 17. TSS, LB, DB
- 18. TSS, LB, SEQ1
- 19. TSS, LB, no plasmid
- 20. TSS, LB + amp, no plasmid
- 21. TSS, LB + amp, PUC19
- 22. TSS, LB + amp, SEQ1
- 23. TSS, LB + amp, DB
Thursday, June 23
- plates from yesterday worked completely as expected
Plate 6:
Plate 15:
- overnight culture in amp grew
- today:
- glycerol stock of DA
- overnight cultures of DB, sEQ1 from plates
- gel of PCR from last night
- DNA extraction 2x (elution)- verified using nanodrop, did not get enough to be successful
- another overnight PCR using different settings
- designed new primers for casA-E + cas3
Friday, June 24
-
- dna extraction using spin method (DB, SEQ1)
-
- went through hassle of ordering new cas primers from IDT
- ordered a pair of primers for each cas gene-this way we can customize and perhaps pcr out in sections
Saturday, June 25
- transformations of DA, DB, and Seq1 in the bb ampr vector into tsp and neb was successful
- made overnight culture to miniprep tomorrow
Sunday, June 26
- lb amp plates
- restriction digest
- wrong enzymes! used EX and EP instead of EX and ES
- didn't linearize plasmid before gel
Monday, June 27
- top 10 lab techniques to learn and love
- dan emphasized that we need to be independent and know these!
- two methods: gingko bioworks (two bricks into desired plasmid) and traditional (EX and ES)
- DA: ES, EX
- Seq1: ES, XP
- PSB1A3: EX, EP
- 1) did not let gel dry completely before removing comb
- 2) too much voltage caused gel deformation
- lesson: don't use it directly! must grow it up first
- ordered more from igem hq
- rehydrated and let culture overnight in tryptic soy broth
- overnight culture of Seq1, DA, DB, and E0840
- Overall message: Not a great day in terms of results, but many tough lessons learned.
Tuesday, June 28
-
- made 4 plates (tryptic soy), as well as 7 more tryptic soy plates
- glycerol stock x 1
- Genomic prep + PCR using primers R1 and R2
- Nanodrop new record! 220ng/ul template DNA
- "Ode to Trinette" Haiku by Joseph Flay
- PCR is hard
- Trinette, you are so thermal
- Thanks for the fun times
- Miniprep of Seq1, DA, DB, E0840
- Nanodrop results (see Kylie's notebook)
- Restriction digests
- Made "master mix" of water, BSA, NEB4 (1x, enough for 30 digests)
- Seq 1: ES, EX
- DA: ES, EX
- DB: ES, EX
- E0840: EP
- Gel (large size)
- Problem: used wrong hyperladder (used I instead of II)
- Successfully isolated: Seq 1 (ES), Seq 1 (EX), DB (EX), E0840 (insert), E0840 (vector)
- Unsuccessful: DA (ES), DA (EX), DB (ES)
- Transformed RA, RB into NEB cells (no control)
- Replated BL21DE3 x 1 and MG1655 x 1 on LB Agar
- Moved plates from 4 degree room to small fridge in lab because they are fixing the room tomorrow (and got rid of some old plates)
- Took inventory (mostly)
- Overnight cultures:
-
- Other notes: Paul Johnson sent us a nice message basically saying that as long as we can justify it, iGEM is here to stay
- (meaning they will keep funding the team in the coming years)).
- We also talked about getting FURI and SOLUR funding for next year's team.
- An REU proposal was discussed, but ultimately abandoned because a majority of the team's students would need to be from outside ASU, which we don't want.
- Overall: people kept very busy, we worked well in teams, however we need to make sure we are really paying attention to what we do- mistakes cost time!
- Tomorrow: plan on ligation, check PCR, run gel for PCR, order primers for halodurans, try restriction of DA again, miniprep and try restriction of RA/RB
Wednesday, June 29
- plates from last night (see pictures):
- LB + AMP + RA
- LB + AMP + RA
- LB + AMP + RB
- LB + AMP + RB
- restriction digest of DA, DB (2 x)
- run gel: CMR product from BH PCR
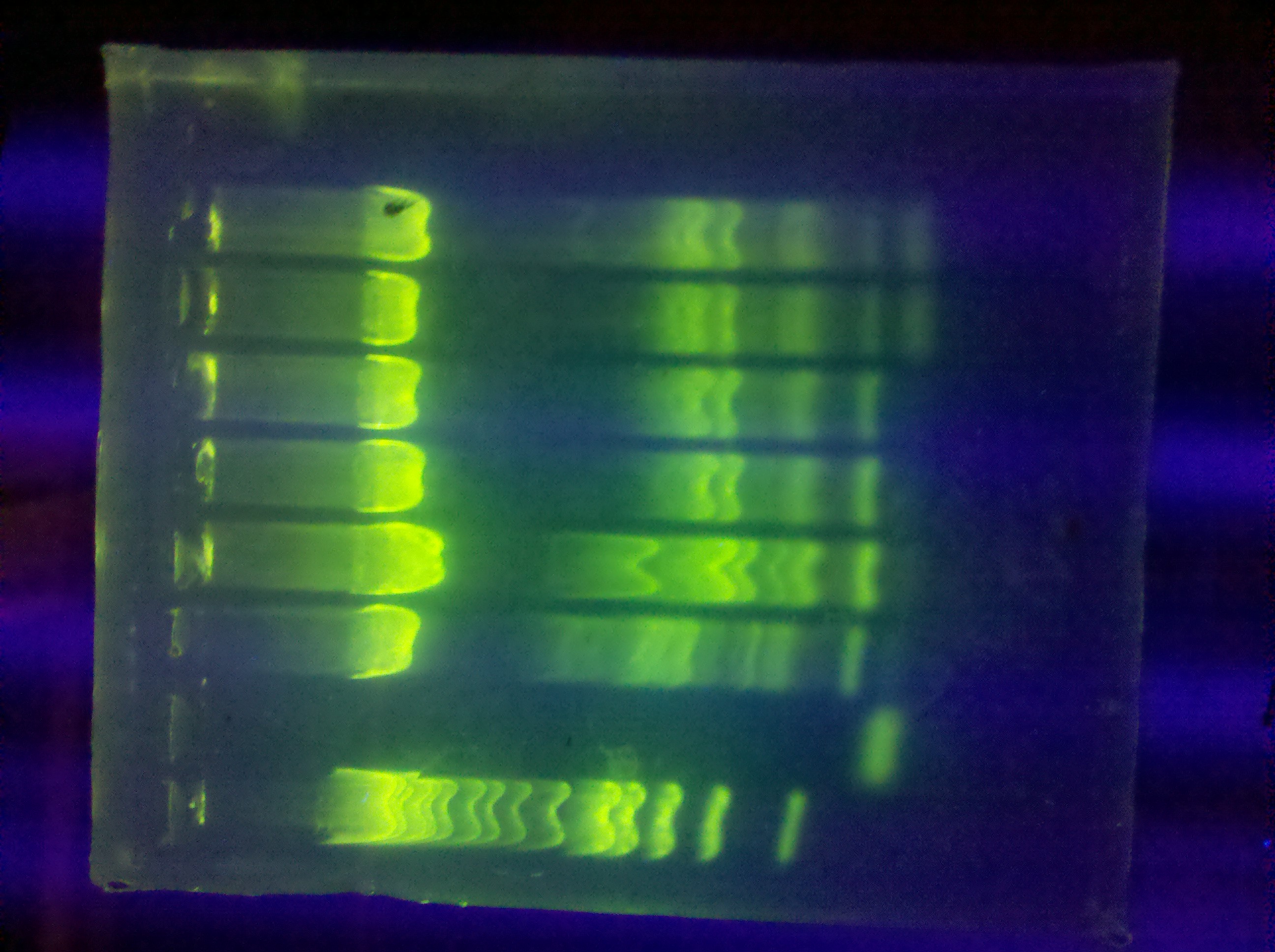
- gel extraction, submitted for sequencing
-
Thursday, June 30
- New primers arrived from IDT for second round of attempts at getting the cas genes out of MG1655
- After successful isolation of what looks like the cmr genes from bacillus halodurans, a second attempt was run overnight
- Got our sequence data from last night for CMR genes: looks like we got what we want
- Gel results:
- Cultures of RA/RB grew well
- Miniprep of RA x2, RB x2
- DA ES EX (2x)
- DB ES XP (2x)
- RA ES EX XP (2x)
- RB ES EX XP (2x)
|