Team:Cornell/Lysis
From 2011.igem.org
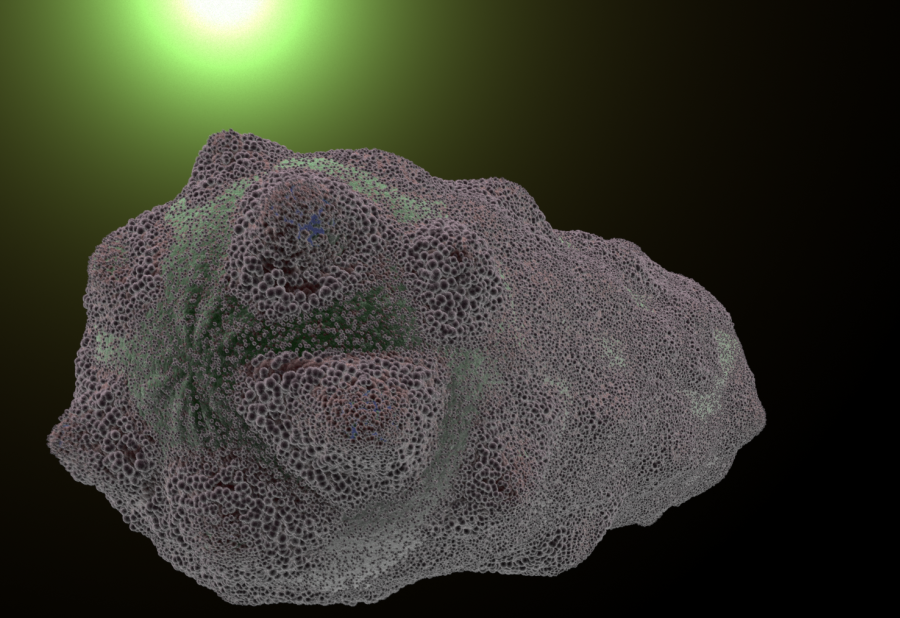
Original Description:
- Beaker of cells floating. Zoom into cells. Watch them float around briefly and zoom close up to one. Show green beams of light
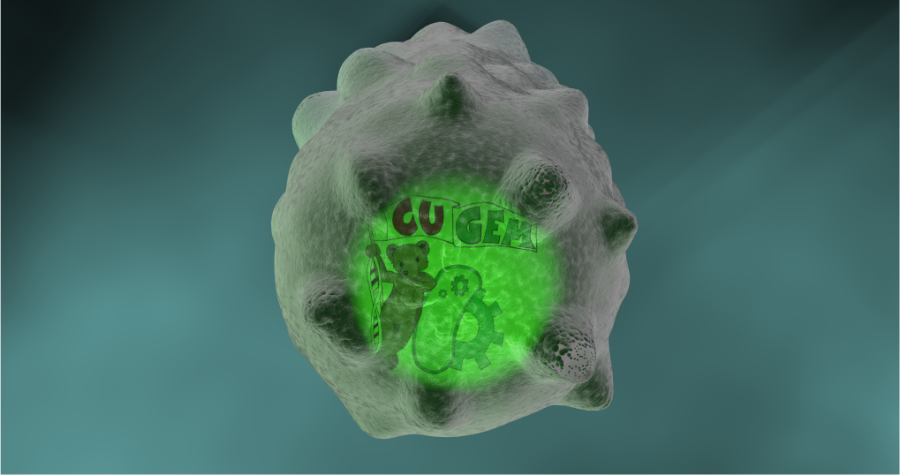
- Zoom out to edge of cell. Small rod-shaped surface proteins pepper surface then aggregate into rings. Cell bulges, inflates, and bursts like a balloon.
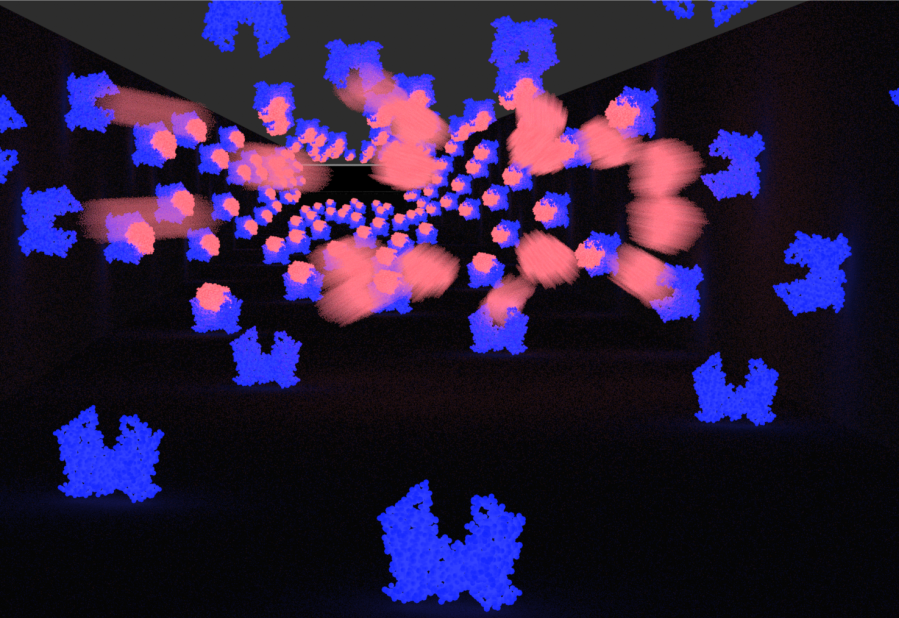
- Zoom out as multiple cells burst.
Interpreted Components:
- E. Coli Model
- Cell Wall - needs to be able to disintegrate.
- Protoplasm - needs maintain shape within cell wall.
- Surface Protein Coating
- Surface Proteins - appearance.
- Coating Pattern
- Explosion Technique
- Bursting Effect.
- Light Source
- Show green color and purpose without being too intrusive.
Summary of Component Development
- Cell Wall
- Created Mesh of E. coli and sculpted it with a mesh of protoplasm underneath in order to maintain consistency in cell wall spacing. Also sculpted morph maps to show wall "gaining holes." At this point in the project, our skills were very limited. We had no viable nice way to explode this.
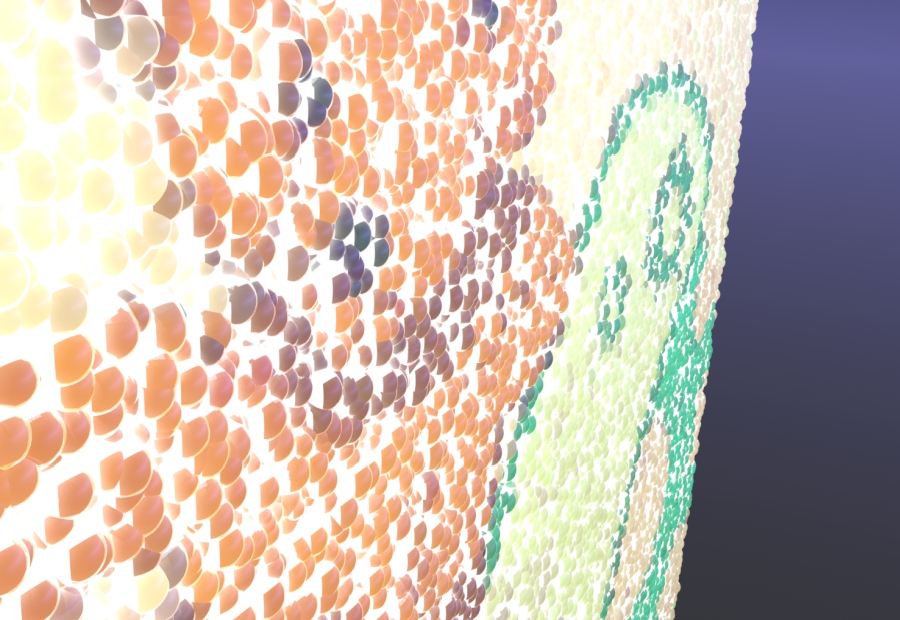
- Replicator Coating - an actual cell wall. Designed by coating the protoplasm mesh with thousands of individual "platelet" meshes. With a surface generator and vertex map deformers, we were able to manipulate it very nicely. Individual platelets also helped give E. coli a more life-like appearance.
- Protoplasm
- Created a mesh of protoplasm underneath cell wall. Worked on appearance. Team leader requested a "wispy" protoplasm; Although cell wall mesh was scratched, protoplasm mesh was very usable and underwent vertex map deformation in sync with its replicator cell wall shell.
- Surface Proteins
- Modeled a stand-in protein model in modo, texturing with a modified version of Yazan Malkosh's electron microscope texture. The team liked appearance of this model.
- Coating Pattern
- We used vertex map deformers for the coating pattern - it took several attempts to get this step right.
- Explosion Technique:
- Experimented with "gooey" and "hard" explosions for meshes, but were not satisfied with the results.
- Experimented with dynamics package. However, this method is far too resource intensive. Thus, we decided that replicator techniques would be more effective.
- Finally, we used a combination of vertex map deformers and morph maps on all replicators and meshes. After several renditions --> We settled on "pop and expand" rendition.
- Beams of Light:
- Started using volumetric direction light sources, but didn't quite like the result. After experimentation, we settled on volumetric point light source with light shift over time. This strategy gives the scene an effective "Sun"
Summary of Scene Progression
For the lysis scene, our team's Modo skill set was still being developed. As a result, this scene involved a good amount of trial and error with various scene rewrites.
- Pure Mesh Attempt. (06/26/2011 - 07/03/2011)
Looked very nice in stills, but scratched before significantly animated.
- 1st Draft - Concept visualization. (07/03/2011 - 07/10/2011)
Created two animation models for the major elements of the scene.
The first illustrated replicator offset explosions with holes in the cell wall. This animation had a positive reception from the team.
The second showcased surface protein patterns and deformations. This version needed some critiquing - discussed the effect of rings vs. globs.
- 2nd Draft - Flagella, Scale Up + Replicator explosion. (07/10/2011 - 07/11/2011)
The draft resulted from an experiment with flagella. Here, the protoplasm mesh is coated with a cell wall replicator that does not deform. So, scaling up the protoplasm resulted in the expansion and "disintegration" of the cell wall. Flagellum was dynamically parented to protoplasm for smooth motion weight gradient. This draft was met with a positive reception, but the team decided to cut the flagellum for next rendition. This rendition also did not include surface proteins, which we had originally planned to include.
- 3rd Draft - 1st Full Assembly. (07/11/2011 - 07/15/2011)
Rebuilt the scene using feedback and lessons from previous renditions.
- Visual Overhaul: Used edited version of Yazan Malkosh's electron microscope texture for cell wall platelets
- Updated and refined vertex map deformers.
- Refined explosion to include organic scaling of bacteria and post-explosion swirling of cell wall platelets and expansion of protoplasm.
This draft met mostly positive reception. Our team leader wanted more prominent "spikey" deformations at holes, and more of a "bursting" explosion. Consensus - camera work made bacteria appear to shrink, which was not our original intention. Additionally, we still needed to implement surface protein layers.
- 4th Draft - 2nd Full Assembly. (07/15/2011 - 07/20/2011)
Refined scene
- Rebuilt and implemented surface protein vertex map deformers. Protein visuals updated.
- Implemented changes to hole deformations. More of a spikey look.
- Modified camera work.
- Refined Explosion - aimed for the effect of a more solid pop and burst.
- Updated green light source
- Changed protoplasm to a less resource intensive material.
This version was welcomed with positive reception. Of our drafts, these results were most satisfactory. Some comments that holes were too spikey, but overall this was a very successful rendition of the scene.
At present, may correct the spikiness of holes, but this is low on the priority list.
- Experimental Methods. (08/08/2011 - present)
- Never implemented in-scene: Post-2nd full assembly, developed a technique for nicer cell wall disintegration around holes. However, this technique would involve a complete rebuild of the scene. We plan to implement this technique for a cleaner disintegration if there is time.
Reception and Review: Satisfactory. Potentially, the scene could use some slight improvements - namely "spikes" and the end protoplasm appearance. Otherwise, the current result is nicely polished.
 "
"