Team:Caltech/Week 8
From 2011.igem.org
| (19 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
==July 31== | ==July 31== | ||
Started a LB+amp overnight culture of the B0034 glycerol stock from the 2010 Caltech iGEM Team<br/> | Started a LB+amp overnight culture of the B0034 glycerol stock from the 2010 Caltech iGEM Team<br/> | ||
| + | ===Results=== | ||
| + | Mass spectrometry did not run correctly; samples did not ionize; must rerun | ||
| + | |||
==August 1== | ==August 1== | ||
PCR pSB3K3 and pNT003 insert with the new primers.<br/> | PCR pSB3K3 and pNT003 insert with the new primers.<br/> | ||
Gel extract the pNT002 insert from the July 29 PCR<br/> | Gel extract the pNT002 insert from the July 29 PCR<br/> | ||
| + | Standard Assembly Digest: K123001, B0014, B0034, R0010, pSB4A5 <br/> | ||
Minimal media transfers<br/> | Minimal media transfers<br/> | ||
| - | Transformation of pUC19 into competent cells for cell dry weight experiment<br/> | + | Transformation and plating of pUC19 into competent cells for cell dry weight experiment<br/> |
| + | Overnight culture of B0034 glycerol stock<br/> | ||
| + | Overnight ligation of pNT002 insert and PSB3K3 backbone<br/> | ||
| + | Restriction digest of parts for pNT002 standard assembly and overnight ligation <br/> | ||
===Results=== | ===Results=== | ||
| - | The enrichment culture plates show no visible growth yet.<br/> | + | No colonies visible on the pUC19-transformed cells<br/> |
| + | <p>The enrichment culture plates show no visible growth yet.</p> | ||
| + | Gel showed that the terminator, RBS, and lac promoter did not digest for standard assembly. <br/> | ||
| + | Gel Extractions of July 29 PCR of pNT002 and pNT003 inserts | ||
<table border="1"> | <table border="1"> | ||
<tr> | <tr> | ||
| Line 25: | Line 35: | ||
<td>12.5</td> | <td>12.5</td> | ||
</tr> | </tr> | ||
| - | |||
</table> | </table> | ||
| - | |||
==August 2== | ==August 2== | ||
Gibson assemble pNT002 using the gel extracted insert and linearized pSB3K3<br/> | Gibson assemble pNT002 using the gel extracted insert and linearized pSB3K3<br/> | ||
Gibson assemble pNT003 using the gel extracted insert and linearized pSB4A5<br/> | Gibson assemble pNT003 using the gel extracted insert and linearized pSB4A5<br/> | ||
| + | Transform Gibson reactions into our XL-10 cells and Emzo's top ten cells<br/> | ||
| + | Replating of more pUC19-transformed competent cells and non-transformed competent cells<br/> | ||
| + | Standard Assembly Digest- K12300, B1004, R0010, B0034, along with the destination plasmids.<br/> | ||
| + | Run gel of overnight pNT002 ligations<br/> | ||
| + | Transformed pNT002 insert+ pSB3K3 ligation<br/> | ||
| + | ===Results=== | ||
| + | <gallery> | ||
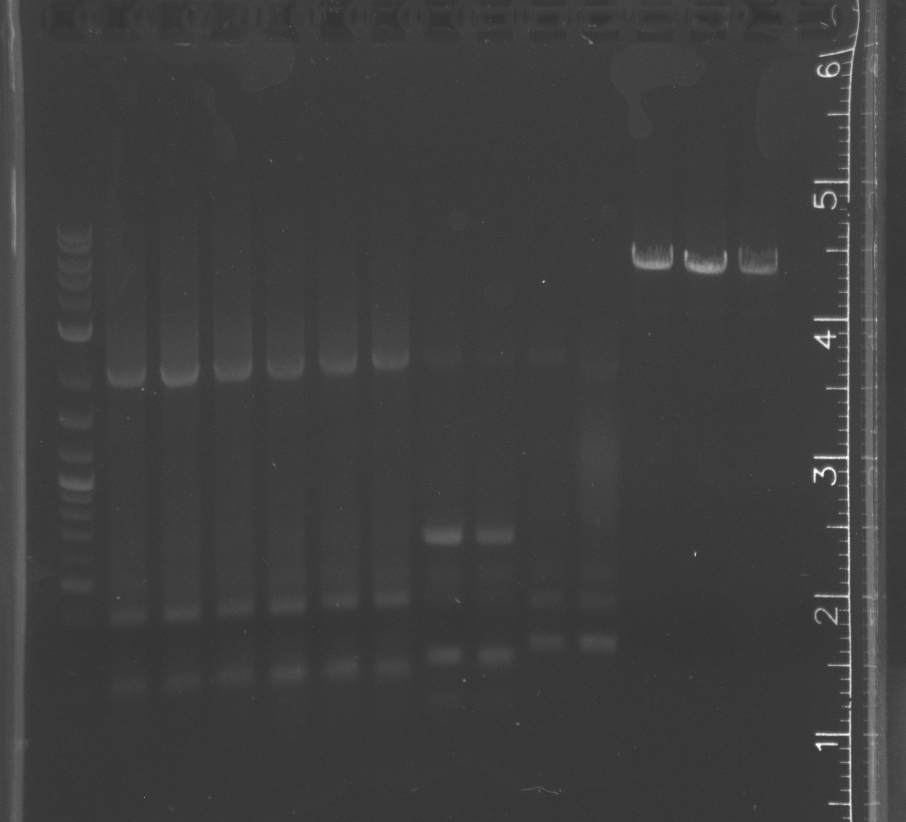
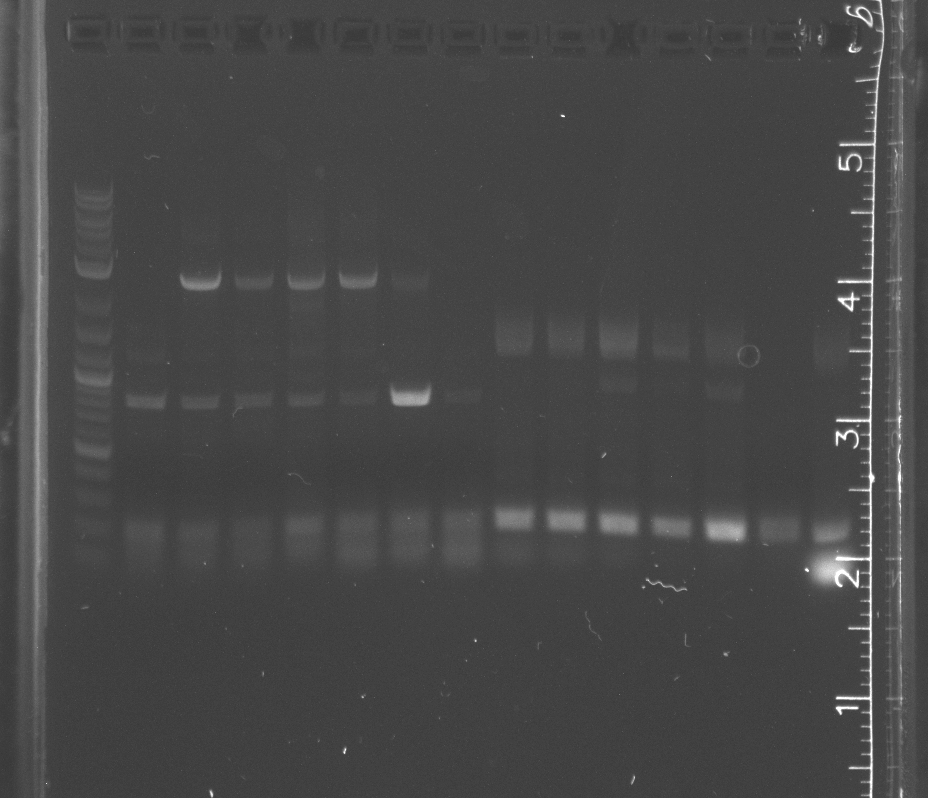
| + | File:8-2pcrgel1.jpg|lane 1 NEB 2-log ladder, 2-4 pNT003 insert, 5-7 pNT003 insert with PIPE primers, 8-13 pSB3K3, 14-15 pETDEST53 | ||
| + | File:8-2pcrgel2.jpg|lane 1 NEB 2-log ladder, 2-4 pNT003 insert, 5-7 pNT003 inset with PIPE primers, 8-9 pNT003 insert, 10-11 pNT003 inset with PIPE primers, 12-15 pETDEST53 | ||
| + | </gallery> | ||
| + | <p>pNT002 ligation showed band on gel</p> | ||
| + | <table border="1"> | ||
| + | <tr> | ||
| + | <th>Part</th> | ||
| + | <th>Concentration (ng/ul)</th> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pSB3k3-1</td> | ||
| + | <td>23.8</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pSB3K3-2</td> | ||
| + | <td>29.9</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pETDEST53</td> | ||
| + | <td>90.9</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | ==August 3== | ||
| + | Colony PCR of Gibson transforms<br/> | ||
| + | Another assembly digest with gel extraction.<br/> | ||
| + | Emzo's protocol for traditional assembly of PCR'd pNT002 insert and pSB3K3 plasmid<br/> | ||
| + | ===Results=== | ||
| + | <table border="1"> | ||
| + | <tr> | ||
| + | <th>Plate</th> | ||
| + | <th>number of colonies</th> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pNT002 + top ten</td> | ||
| + | <td>55</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pNT002 - top ten</td> | ||
| + | <td>43</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pNT002 +XL10 gold</td> | ||
| + | <td>180</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pNT002 - XL10 gold</td> | ||
| + | <td>400</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pNT003 + top ten</td> | ||
| + | <td>14</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pNT003 - top ten</td> | ||
| + | <td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pNT003 +XL10 gold</td> | ||
| + | <td>0</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pNT003 - XL10 gold</td> | ||
| + | <td>7</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | Only the enzyme and the vector had correct band lengths on the gel. We extracted them and nanodropped, but concentrations too low.<br/> | ||
| + | <table border="1"> | ||
| + | <tr> | ||
| + | <th>Part</th> | ||
| + | <th>Concentration (ng/ul)</th> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>K123001</td> | ||
| + | <td>2.4</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pSB4A5</td> | ||
| + | <td>6.2</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | |||
| + | ==August 4== | ||
| + | Ran gel of colony pcr from yesterday<br/> | ||
| + | Ran two separate gels for standard assembly according to the number of base pairs each had. <br/> | ||
| + | The first 1.3% agarose gel had a 100 bp ladder with R0010 (200 bp), B0014 (95 bp), and B0034 (12 bp).<br/> | ||
| + | The second 1% agarose gel had a 2-log ladder with K123001 (1284 bp) and pSB4A5 (3395 bp).<br/> | ||
| + | Grow two overnight cultures of 175mL LB-chlor in preparation of dry cell experiment | ||
| + | ===Results=== | ||
| + | Some of the colony PCR have insert amplifications of the correct length. Will send some off for sequencing tomorrow.<br/> | ||
| + | <gallery> | ||
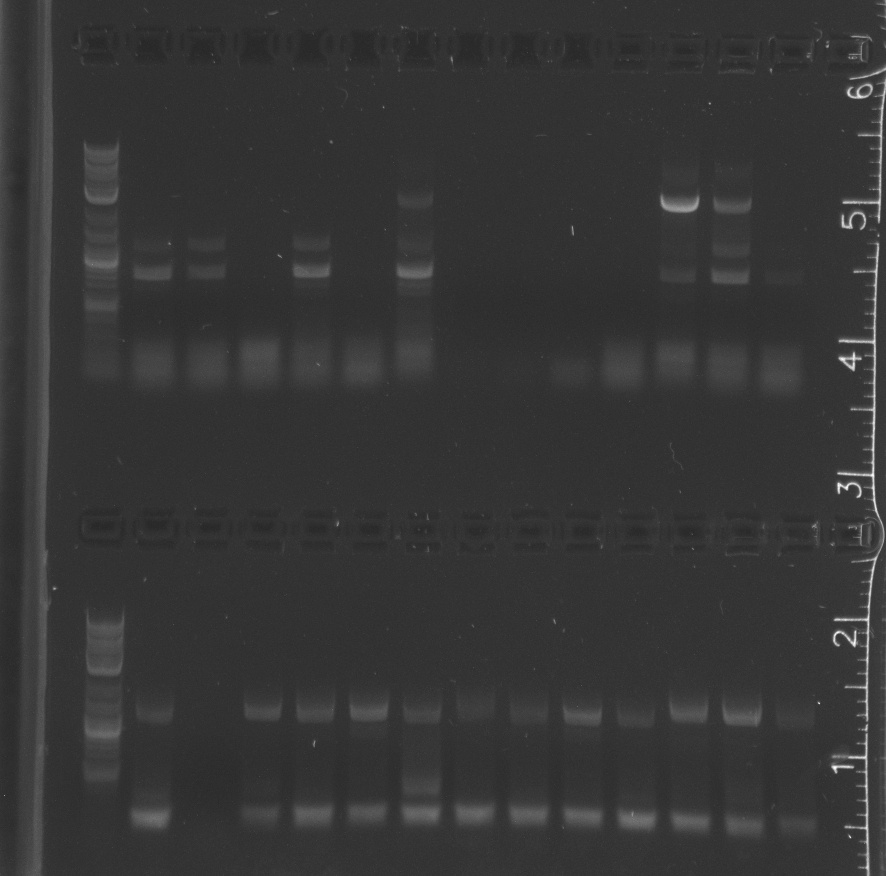
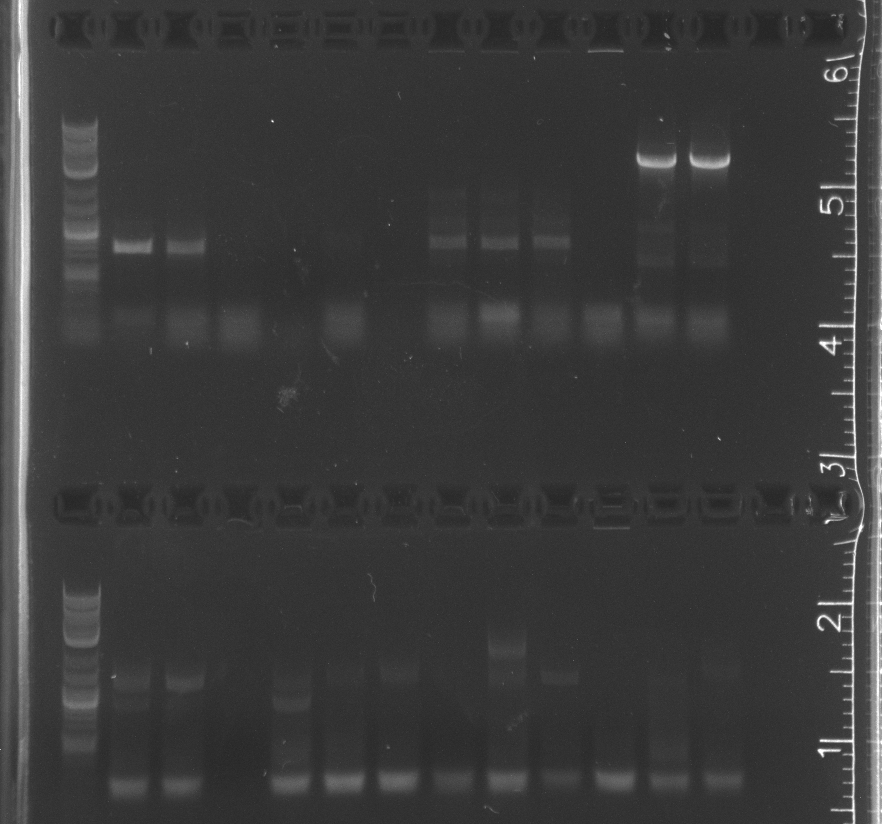
| + | File:8-4colonypcr002.jpg|upper comb lane 1 NEB 2-log ladder, 2-14 pNT002 colonies 1-13 vector amplification; lower comb 1 NEB 2-log ladder, 2-14 pNT002 colonies 1-13 insert amplification | ||
| + | File:8-4colonypcr002gel2.jpg|lane 1 NEB 2-log ladder, 2-8 pNT002 colonies 14-20 vector amplification, 9-15 pNT002 colonies 14-20 insert amplifications | ||
| + | File:8-4colonypcr003.jpg|upper comb lane 1 NEB 2-log ladder, 2-13 pNT003 colonies 1-12 vector amplification; lower comb 1 NEB 2-log ladder, 2-13 pNT003 colonies 1-12 insert amplification | ||
| + | </gallery> | ||
| + | No growth from traditional assembly on self ligation control or experimental plate<br/> | ||
| + | Blue light showed bands for all the parts, but again the smaller parts had the wrong band lengths.<br/> | ||
| + | ==August 5== | ||
| + | Miniprepped colonies from Gibson colony pcr (pNT003-2,8,11,12 and pNT002-6,11,12,14)<br/> | ||
| + | Sent pNT002-14 and pNT003-2,8,11,12 off for sequencing as the others were too dilute<br/> | ||
| + | PCR 16s sequences<br/> | ||
| + | Rerun p450 degradation assay for GCMS analysis<br/> | ||
| + | Begin growing biofilms in 96-well plate and Erlenmeyer flasks with pipette tips, glass beads, and wood chips with different OD's of XL-10 | ||
| + | Assembled DDT gene and transformed into XL-10s | ||
| + | ===Results=== | ||
| + | <table border="1"> | ||
| + | <tr> | ||
| + | <th>Miniprep name</th> | ||
| + | <th>Concentration (ng/ul)</th> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pNT003-2</td> | ||
| + | <td>45.3</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pNT003-8</td> | ||
| + | <td>32.3</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pNT003-11</td> | ||
| + | <td>33.1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pNT003-12</td> | ||
| + | <td>33.1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pNT002-6</td> | ||
| + | <td>9.2</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pNT002-11</td> | ||
| + | <td>12.4</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pNT002-12</td> | ||
| + | <td>12.1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pNT002-14</td> | ||
| + | <td>78.1</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | The gel for 16s had no bands in any of the control or sample lanes. | ||
| + | The transformations for the DDT gene yielded no colonies. Will redo assembly. | ||
| + | ==August 6== | ||
| + | Transfer 96-well plate and flasks to 37°C incubator | ||
}} | }} | ||
Latest revision as of 17:19, 9 August 2011
|
Project |
July 31Started a LB+amp overnight culture of the B0034 glycerol stock from the 2010 Caltech iGEM Team ResultsMass spectrometry did not run correctly; samples did not ionize; must rerun August 1PCR pSB3K3 and pNT003 insert with the new primers. ResultsNo colonies visible on the pUC19-transformed cells The enrichment culture plates show no visible growth yet. Gel showed that the terminator, RBS, and lac promoter did not digest for standard assembly.
August 2Gibson assemble pNT002 using the gel extracted insert and linearized pSB3K3 ResultspNT002 ligation showed band on gel
August 3Colony PCR of Gibson transforms Results
Only the enzyme and the vector had correct band lengths on the gel. We extracted them and nanodropped, but concentrations too low.
August 4Ran gel of colony pcr from yesterday ResultsSome of the colony PCR have insert amplifications of the correct length. Will send some off for sequencing tomorrow. No growth from traditional assembly on self ligation control or experimental plate August 5Miniprepped colonies from Gibson colony pcr (pNT003-2,8,11,12 and pNT002-6,11,12,14) Results
The gel for 16s had no bands in any of the control or sample lanes. The transformations for the DDT gene yielded no colonies. Will redo assembly. August 6Transfer 96-well plate and flasks to 37°C incubator
|
 "
"