A Proposed New Standard for iGEM Human Practices: The IRB Approved Survey
Traditionally, iGEM Human Practice efforts have fallen under 3 realms:
- An Essay on Bioethics & Synthetic Biology (e.g. HP award in Indiana)
- A tutorial or seminar for Students (e.g. 2011 Americas Regional Jamboree Winners Team University of Washington)
- A survey for other iGEM team members or students.
Another trend of human practices projects in iGEM is that these projects tend to focus on the field of synthetic biology as a whole. While knowledge generated from such efforts are useful and interesting, they do not specifically address the difficulty specific iGEM projects might face once they are being applied in the real world. In other words, many human practices projects are only loosely tied to the iGEM projects they belong to.
The 2011 Johns Hopkins iGEM team has taken steps to address these problems. First, we have crafted a Human Practices survey that has successfully passed through the the Institutional Review Board (IRB), the standard regulatory body for human subjects research. Second, the focus of the survey has been geared towards the actualization of the team’s VitaYeast project. The survey was constructed in such a way that would allow the team to find potential difficulties VitaYeast may face, should it ever reach the mass market. The outcome is a growing collection of real and eventually publishable data about VitaBread collected under the banner of the Johns Hopkins University School of Medicine.
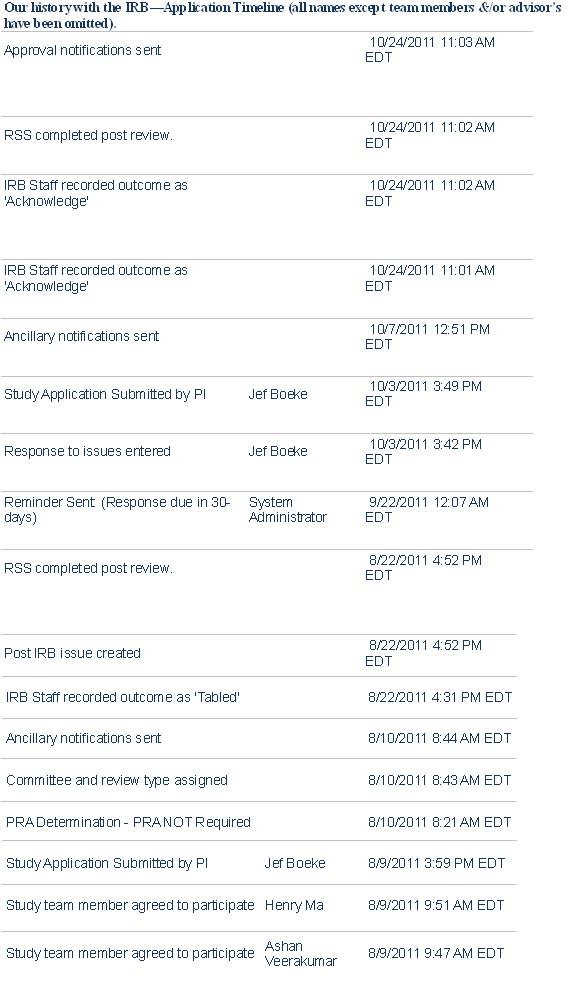
The Johns Hopkins iGEM Team’s Road to IRB Approval: A Guide for Future Teams
Quick Facts on The JHU iGEM Human Practices Survey:
- It is an anonymous 16 part questionnaire on the perception of GM foods including two demographic questions.
- It is distributed at two Baltimore markets to ordinary Baltimore Citizens
- It is approved as an Exempt Study by the Johns Hopkins University School of Medicine Institutional Review Board (IRB).
- It aims to gauge the immediate appeal of Vitabread in relation to other GM and Non GM varieties
- It aims to find an approximate ‘acceptable percentage’ of GM content in a recipe.
Some FAQs
What is an Institutional Review Board (IRB)?
An IRB is essentially an institution’s own Bioethics board that reviews, helps refine, and green-lights research projects on human subjects research. It is there to make sure that no human subject’s rights will be violated in any shape or form by affiliated studies. For a more detailed explanation, see the [http://www.hopkinsmedicine.org/institutional_review_board/ Hopkins Medicine IRB Website].
Do you have to get IRB approval to do a survey?
[http://printfu.org/read/welcome-to-cornell-university-s-institutional-review-board-irb--7bd9.html?f=1qeYpurpn6Wih-SUpOGunK6nh8PU4sjj1cqQ5tiOt9Xn28rY25a64tHb1eTc1-jfV-3-34-_0-fczuTn3dfj1NbZhb7U7M7Z34Wy4crg2Iadtreuj6STooqg46ybqJba6Y-g36mnlq6Kz-Xl3dvktsXS0dGRsdiumZ-S2Iup56Csop-O1-rZ5KKUn-ng5aLP58-Tz97o09nU0Z7XzeOjyuTQ2tnU5Nnnl7jk583X2dman5Xj2OrN493ZlaSZt8aomp-Vrd_m1-Pextyg2dLaiLDq You do not explicitly require IRB approval] to conduct human practices research for iGEM. If you do not seek IRB approval and intend to publish the collected data, you may have to disclose this fact to the potential publisher first. Depending on your institution, you may be required to make other disclosures as well (e.g. to the participants themselves that your project was not IRB approved).
So why seek IRB approval in iGEM?
Seeing as synthetic biology is one of the [http://bioethics.gov/cms/studies primary focuses of modern bioethics], the field is inherently controversial and therefore subject to intense scrutiny. IRB approval is the easiest way to certify a project as ‘ethically clean’ for the simple reason that a bioethics board has approved it. It raises the standard of the project and makes it more acceptable to the general public. For example, if all iGEM projects had IRB approved elements, the institution as a whole would be easily argued as one that cares about bioethics and aims to remain ethically sound.
Side Benefit: Study Team Members on an IRB Approved Study take short courses on Bioethics (e.g. Conflict of Interest, Human Subjects Research), which allows them to learn more about the field.
How should iGEM teams approach the process of IRB approval?
Design a study to receive IRB exempt status. ‘Exempt’ refers to a type of IRB approval granted without an extended review process. In our case, the IRB took around 2 weeks to review our second application and approve us. To design an exempt study, follow the guidelines of your institution. For viewing purposes, you can view [http://www.hopkinsmedicine.org/institutional_review_board/guidelines_policies/guidelines/exempt_research.html the Hopkins Medicine guidelines].
NOTE: A faculty member is required to submit all final documents.
Sections of the Hopkins Medicine IRB Application (Note omitted sections for those applying for exempt status).
1 - General Information
2 - Study Team Compliance
4 - Exempt Research
6 - Protocol Information
8 - Conflict of Interest
9 - Support Information
10 - Study Location
11 - Sample Size and Source
12 - Participant Information
13 - Recruitment Information
14 - Consent and Waivers
20 - Supplemental Study Documents
24 - Human Biological Samples
32 - SKCCC CRO
34 - Data Confidentiality
35 - Application Documents
Complete Application
Responding to IRB Issues.
We received approval on the second application; clearly, we made mistakes on our first attempt. Keep in mind that all institutions have their own rules, but we would like to provide some advices to future iGEM team seeking IRB approval.
The essential problems with our first application are as follows:
1) We first wanted to pursue a multinational survey by use of our contacts in various countries.
We found it difficult to effectively write a detailed recruitment strategy and receive formal permission from different survey sites for each country. Furthermore, one institution’s IRB cannot make a judgment about what is ‘ethically sound’ in other countries. To do a multinational survey, we would have had to collaborate with endemic institutions and have them pass the study through their own IRB reviews.
2) Entered information often must be repeated in the various sections of an IRB application. Whenever we repeated information, a couple facts would sometimes not match. Although this mistake seems trivial, it is reportedly one of the most common problems in all the applications our IRB receives.
We eventually solved these issues by aggressively downsizing our application from a multinational study to a study involving two Baltimore markets. The smaller size of the study allowed us to easily obtain the required permission letters from the appropriate authorities and write a detailed study protocol. We also took greater care in proofreading our material before submitting. After the competition, we will amend the study to include more sites and survey methods.
Conclusion
Start with a small study and aim to get an IRB exemption. They can be amended to add new sites, study team members, etc.
Write a detailed human subject recruitment protocol and get formal permission from each of the sites you plan on conducting your study.
Proofread the application multiple times to make sure to everything matches. Treat the iGEM human subjects research activities like another lab experiment and apply the same amount of care and diligence to your work.
 "
"