Team:Paris Bettencourt/Experiments/Methodologies/Tecan
From 2011.igem.org
(→Nanotube experiment) |
(→Protocol) |
||
| (18 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{:Team:Paris_Bettencourt/tpl_test}} | {{:Team:Paris_Bettencourt/tpl_test}} | ||
| - | What we call the | + | =Kinetics= |
| + | |||
| + | What we call the Tecan® is the Infinite 200 PRO multimode reader from Tecan®. | ||
[[File:Tecan_Picture_Paris.jpg|right]] | [[File:Tecan_Picture_Paris.jpg|right]] | ||
| - | This machine can read | + | This machine can read fluorescence and absorbance in a 96 well plate, within cycles that are programmed to be repeated, and the kinetics can last for hours. It allows to look at evolution of fluorescence during all the growth of a bacterial colony.<br> |
| - | In our case as the receiver and the emitter of each of our system have specific fluorescence, we tuned the | + | In our case as the receiver and the emitter of each of our system have specific fluorescence, we tuned the Tecan® to measure fluorescence every 5 or 10 minutes for 3 to 12 hours depending on what we were studying. To characterize a part, we usually do an overnight experiment. To test wether nanotubes are forming or not, 3 to 4 hours are enough since we need <i>B.subtilis</i> to be in exponential phase.<br> |
| - | The | + | The sensitivity of the Tecan® allows precise quantification of the fluorescence emitted by our various designs. |
==Nanotube experiment== | ==Nanotube experiment== | ||
| - | What we want to measure | + | ===Optimization=== |
| - | *cells in rich media (LB) | + | |
| - | *cells in rich media, with on the bottom of the well a thin layer of LB agar, | + | What we want to measure is the appearance of fluorescence in receiver system (switch off -> on). But to switch the receiver system on, we needed to make sure we could reproduce the conditions of nanotubes formation in the 200 microliters wells of the 96 plate. We therefore first tested the growth of <i>Bacillus subtilis</i> in three different conditions to see if it could grow normally : |
| + | *cells in rich media (LB), concentrated at the bottom of the well by centrifugation | ||
| + | *cells in rich media (LB), with on the bottom of the well a thin layer of LB agar, concentrated by centrifugation again | ||
*cells in rich media without centrifugation | *cells in rich media without centrifugation | ||
| - | Our controls were LB without bacteria, and LB agar and LB without bacteria. | + | Our negative controls were LB without bacteria, and LB agar and LB without bacteria. |
| - | We tuned the Tecan for | + | We fine tuned the Tecan sensitivity for each experiments. At the end of experiments we resuspend the cells and measure the optical density. We are hence able to compare the cells growth in each conditions. |
[[File:Curves_GFP+_Paris.jpg|center|800px]] | [[File:Curves_GFP+_Paris.jpg|center|800px]] | ||
| - | + | As you can see from the experiment result's, the LB agar is not important for the cell's growth. | |
| + | |||
| + | ===Tested conditions=== | ||
| + | |||
| + | Once the experimental set up optimized, we systematically tested in triplicate our designs with different ratios of emitter versus receiver strains. | ||
| - | |||
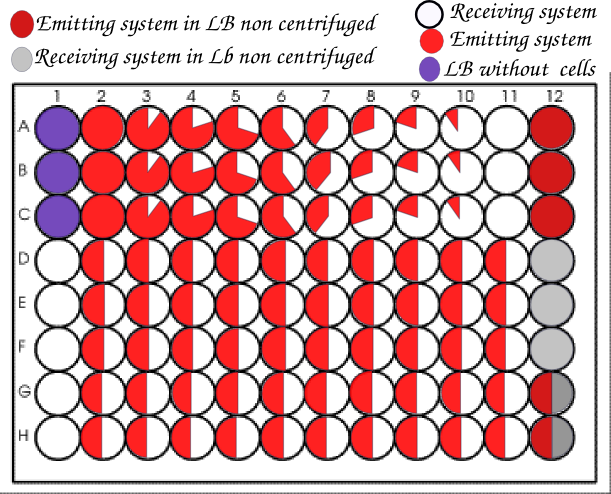
We usually organized our plate as below.<br> | We usually organized our plate as below.<br> | ||
[[File:Tecan_nanotube_plate_Paris.jpg|center|700px]] | [[File:Tecan_nanotube_plate_Paris.jpg|center|700px]] | ||
| - | + | As the ratio 1:1 emitter/receiver seemed to be the most favorable according the original paper, we decided to have most wells in these optimal conditions to increase our chances of observing nanotubes. | |
| - | + | ||
| - | + | ||
<br> | <br> | ||
| + | ===Protocol=== | ||
| + | |||
| + | *let grow the strains in LB until the OD reaches 0.8 (if <i>subtilis</i>) or 0.5 (if <i>coli</i>) | ||
| + | *fill the plate with the different ratios and negative controls | ||
| + | *measure the OD in the whole plate | ||
| + | *centrifuge at 1500rpm for 7 minutes | ||
| + | *resuspend some wells for negative controls | ||
| + | *let them in the Tecan® with a measure of fluorescence of interest every 5mn for 4 hours. | ||
| + | *when the kinetic is over resuspend the cells | ||
| + | *measure the OD again | ||
==Characterisation experiment== | ==Characterisation experiment== | ||
| + | All our system were designed for the emitter cell to express one fluorescence and its receiver an other one. Moreover most of the time the "answer" of the receiver system when getting the signal is to express a new fluorescence. | ||
| + | Therefore most of our parts rely on fluorecent reporter, and we could realize some of our characterisation, with that machine. | ||
| + | |||
| + | ===Protocol=== | ||
| + | |||
| + | *let the strains grow in rich liquid media(LB) until the OD reaches 0.2 | ||
| + | *dilute them with LB with or whithout inducer/inhibitor | ||
| + | *fill the plate wiith those solutions | ||
| + | *add mineral oil on the top of it to avoid evaporation | ||
| + | *tune the tecan to realise OD and fluorescence measures every 10minute for 12 hours | ||
| - | |||
| - | |||
<br><br> | <br><br> | ||
Latest revision as of 03:47, 29 October 2011

Contents |
Kinetics
What we call the Tecan® is the Infinite 200 PRO multimode reader from Tecan®.
This machine can read fluorescence and absorbance in a 96 well plate, within cycles that are programmed to be repeated, and the kinetics can last for hours. It allows to look at evolution of fluorescence during all the growth of a bacterial colony.
In our case as the receiver and the emitter of each of our system have specific fluorescence, we tuned the Tecan® to measure fluorescence every 5 or 10 minutes for 3 to 12 hours depending on what we were studying. To characterize a part, we usually do an overnight experiment. To test wether nanotubes are forming or not, 3 to 4 hours are enough since we need B.subtilis to be in exponential phase.
The sensitivity of the Tecan® allows precise quantification of the fluorescence emitted by our various designs.
Nanotube experiment
Optimization
What we want to measure is the appearance of fluorescence in receiver system (switch off -> on). But to switch the receiver system on, we needed to make sure we could reproduce the conditions of nanotubes formation in the 200 microliters wells of the 96 plate. We therefore first tested the growth of Bacillus subtilis in three different conditions to see if it could grow normally :
- cells in rich media (LB), concentrated at the bottom of the well by centrifugation
- cells in rich media (LB), with on the bottom of the well a thin layer of LB agar, concentrated by centrifugation again
- cells in rich media without centrifugation
Our negative controls were LB without bacteria, and LB agar and LB without bacteria. We fine tuned the Tecan sensitivity for each experiments. At the end of experiments we resuspend the cells and measure the optical density. We are hence able to compare the cells growth in each conditions.
As you can see from the experiment result's, the LB agar is not important for the cell's growth.
Tested conditions
Once the experimental set up optimized, we systematically tested in triplicate our designs with different ratios of emitter versus receiver strains.
We usually organized our plate as below.
As the ratio 1:1 emitter/receiver seemed to be the most favorable according the original paper, we decided to have most wells in these optimal conditions to increase our chances of observing nanotubes.
Protocol
- let grow the strains in LB until the OD reaches 0.8 (if subtilis) or 0.5 (if coli)
- fill the plate with the different ratios and negative controls
- measure the OD in the whole plate
- centrifuge at 1500rpm for 7 minutes
- resuspend some wells for negative controls
- let them in the Tecan® with a measure of fluorescence of interest every 5mn for 4 hours.
- when the kinetic is over resuspend the cells
- measure the OD again
Characterisation experiment
All our system were designed for the emitter cell to express one fluorescence and its receiver an other one. Moreover most of the time the "answer" of the receiver system when getting the signal is to express a new fluorescence. Therefore most of our parts rely on fluorecent reporter, and we could realize some of our characterisation, with that machine.
Protocol
- let the strains grow in rich liquid media(LB) until the OD reaches 0.2
- dilute them with LB with or whithout inducer/inhibitor
- fill the plate wiith those solutions
- add mineral oil on the top of it to avoid evaporation
- tune the tecan to realise OD and fluorescence measures every 10minute for 12 hours
 "
"