Team:Paris Bettencourt/Experiments/YFP TetR diffusion
From 2011.igem.org
| Line 11: | Line 11: | ||
We test different possibilities : at 37°C or 30°C and concentration of arabinose (0% - 0,1% -0,2%) to deal with protein agregation. | We test different possibilities : at 37°C or 30°C and concentration of arabinose (0% - 0,1% -0,2%) to deal with protein agregation. | ||
</html> | </html> | ||
| - | + | <center> | |
{| border="1" class="wikitable" style="text-align: center;" align="center" | {| border="1" class="wikitable" style="text-align: center;" align="center" | ||
|+tetR:YFP protein fusion : 37°C | |+tetR:YFP protein fusion : 37°C | ||
| Line 30: | Line 30: | ||
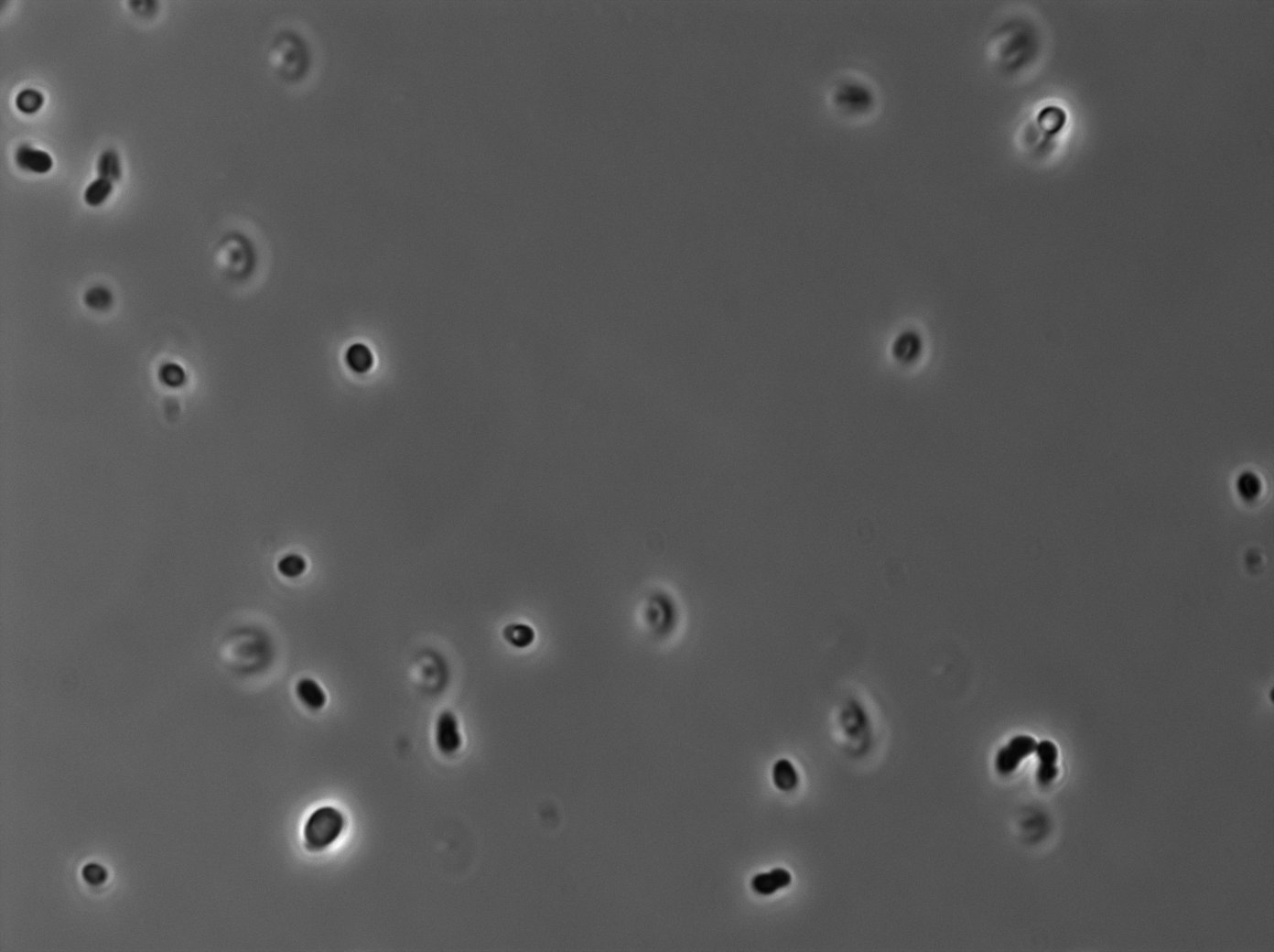
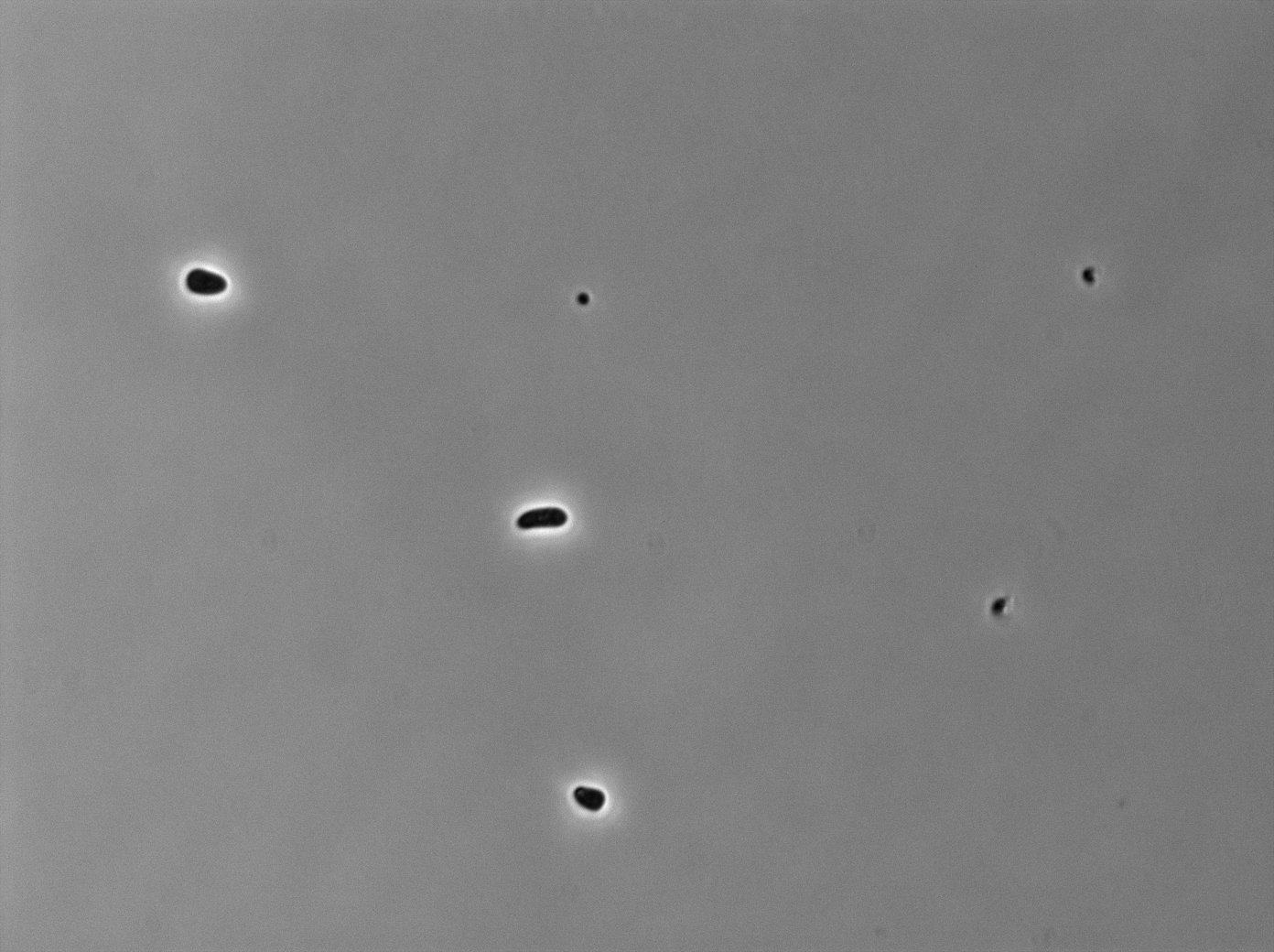
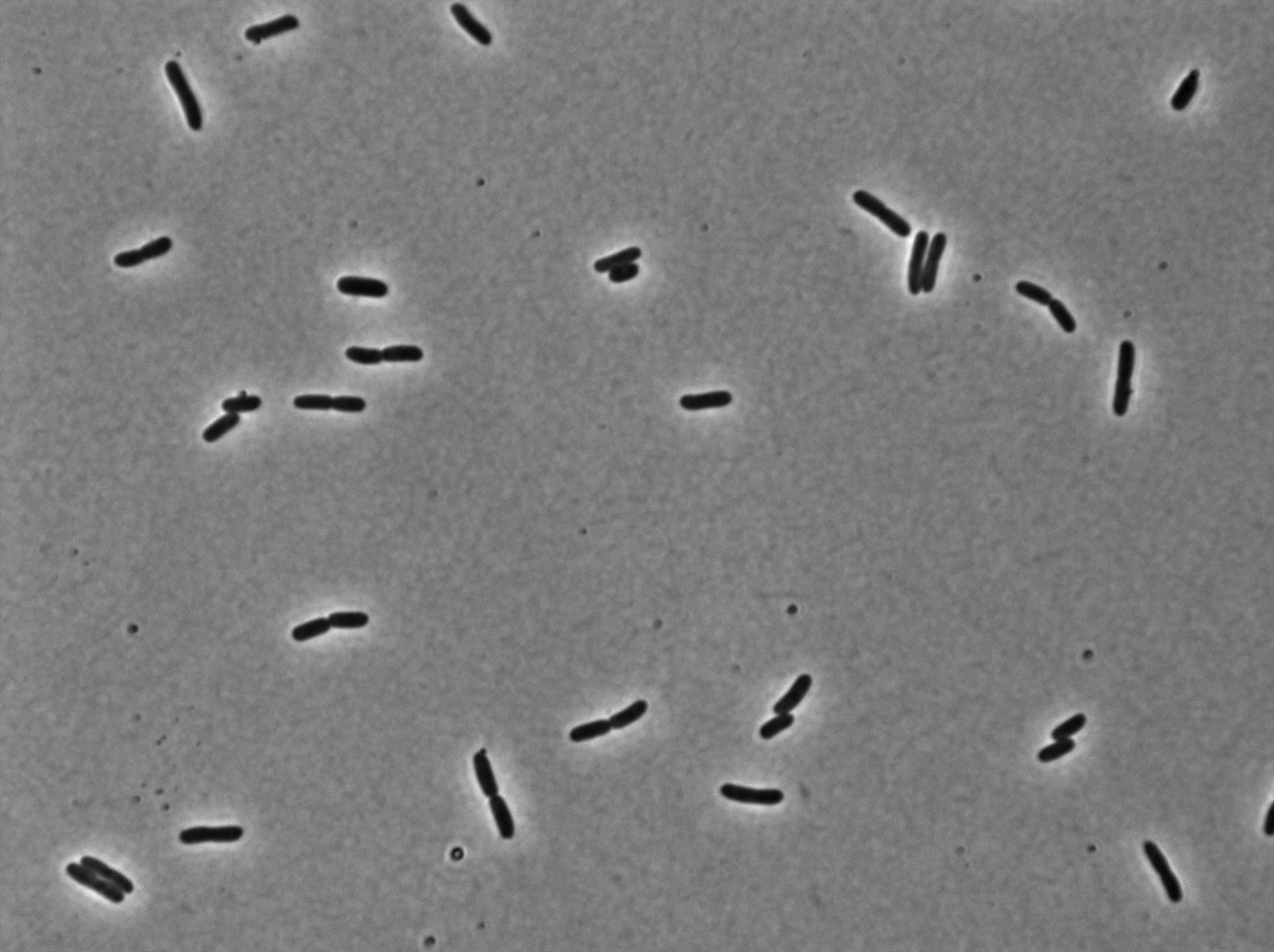
|[[File:TetR02_Trans03.jpg|350px|thumb|center|tetR:YFP / tetO array inducted with 0,2% arabinose on E. Coli from Dave Lane plasmids.]] | |[[File:TetR02_Trans03.jpg|350px|thumb|center|tetR:YFP / tetO array inducted with 0,2% arabinose on E. Coli from Dave Lane plasmids.]] | ||
|[[File:TetR02_YFP03.jpg|350px|thumb|center|tetR:YFP / tetO array inducted with 0,2% arabinose on E. Coli from Dave Lane plasmids.]] | |[[File:TetR02_YFP03.jpg|350px|thumb|center|tetR:YFP / tetO array inducted with 0,2% arabinose on E. Coli from Dave Lane plasmids.]] | ||
| - | |} | + | |}</center> |
<html> | <html> | ||
Revision as of 14:37, 12 September 2011

Experiments of the YFP concentration design
The planning of the experiments is the following : first we have tested the strains from D. Lane containing YFP:tetR and tetO array. Then we constructed/biobricked the YFP:tetR and tetO array system. To finish with the microscopy step and results of this proof of concept between B. subtilis and B. subtilis / E. coli.
Testing the YFP:tetR strains from D. Lane
In the article [1], E. coli strains are growing at 20°C to avoid protein agregation but the problem is that nanotube between B. subtilis has been only proved to exist at 37°C. We test different possibilities : at 37°C or 30°C and concentration of arabinose (0% - 0,1% -0,2%) to deal with protein agregation.More pictures and information on the notebook [2].
Biobricked system construction
Results and microscopy of the proof of concept
In the emittor cell (B. Subtilis), we have inserted a expressive system for the YFP:tetR. It contains the promoter pVeg, the RBS for B. Subtilis and the YFP:tetR protein. Production of YFP:tetR will diffuse throught the nanotube to the receiver cell.
In the receiver cell (B. Subtilis or E. Coli), there is the tetO array where diffused YFP:tetR will concentrate. The YFP is the monitor of the signal.
The principle of the design is summed up in the image below

Fig1: Schematics of the YFP concentration design
 "
"