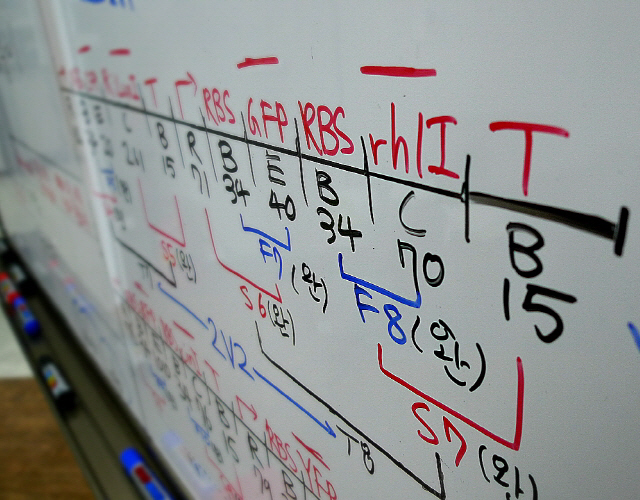Team:KAIST-Korea/Notebook/Week1
From 2011.igem.org
Week 1
6/6 Monday
Jeonghyun, Sohyun, Seoneung, Jijeong, Joonhyuk, Youngjun
10 a.m. - 10 p.m. @ KI building
We have done three things today.
First, we discussed on our biobrick design and has made quite detail plan how to build the biobricks to reach our final goal - making Ecasso. Jeonghyun explained what we were under the illusion on biobrick design, i.e., about prefix and sufix of standardized biobricks, which made all of us surprised (the detail attached). Based on the biobrick concept, we could solve several problems on the biobrick design (the detail attached).
Second, Nambin made a presentation to summarize what the dry lab had done - modeling of the project, Ecasso. The presentation showed modeling of Ecasso in the various conditions and concluded it's relisable to make Ecasso as we expect.
Third, we prepared for the experiments that we're gonna begin tomorrow. Under Jeonghyun's guidance, we've prepared LB media plates containing antibiotics - kanamycin, ampicilin, or both kanamycin and ampicilin (the protocol attached).
Now, what we need to do are labeling on the lab. drawers and tools such as the magnetic stirrer and buying more stuffs in need (the list attached), organizing protocols for experiments on lab notes, and making our own slogan. As mentioned, we're gonna start the experiments tomorrow, amplifying the distributed biobricks by diluting the biobricks, transforming them to the E. coli, and culturing them on the media we've prepared today.
6/7 Tuesday
All dry lab members
@ Library
In order to best approximate the real system of engineered E. coli, we referred to many resources available to us including database on the internet, books on synthetic biology, and textbooks on system design recommended by a professor in our department studying the subject. The assumptions made in the model as well as the references considered are listed under each page in the modeling page.
All wet lab members
@ KIB
So far, we've made the detail plan how to build our new biobricks and prepared the LB media with antibiotics required to amplify the biobricks we need in the project.
Today, we've performed experiments to amplify the biobricks we're gonna use. First, we took the biobricks we need. We could get total 25 biobricks required for our project from the biobrick plate kits that is distributed. Next, we transformed the biobricks to the competent E. coli cells and cultured them. Finally, we spread the E. coli cells on the prepared LB media and put them in incubator. In summary, we've taken the required biobricks, transformed tham into the competent cells, and put them in incubator to amplify the biobricks.
Now, what we need to do are checking if the colonies are formed properly in all the plates of the biobricks, performing experiment again if there are any plates that have no colonies, and buying things in need (attached list).
6/8 Wednesday
All wet lab members
1 p.m. - 6 p.m. @ KIB
By yesterday, we had cultured competent cells that were transformed with the biobricks we’re going to use. We prepared the antibiotic-containing LB media first, took up the biobrick-containing plasmids from the distributed biobrick kits, transformed the plasmids into the competent cells by heat-shock, spread the cells on the media plates, and put the plates in the incubator to culture the transformed cells. The used competent cells, which had been culturing, were expected to have taken up the biobrick plasmids.
Today, we’ve done two things. First, we performed an experiment in order to examine if the transformation of the plasmids is achieved properly. We checked the formation of the colonies on each media plate first, and picked up the colonies and put them into the liquid media (protocol attached). The plasmids will be extracted from the cultured cells in the liquid media. Next, we had discussion on the progress of the research and the logo design with the dry lab members and the designer. We first saw the presentation of dry lab, which was about the modeling of our project (content attached), and then determined the logo design that best suits our project among the 9 candidates brought by the designer. So, we’ve prepared the cells that will be used for the extraction of the plasmids and determined the best logo design for the project.
Tomorrow, we’re going to start mini-prep experiment to extract the biobrick plasmids from the cells that are growing in the liquid media. We are thinking about finishing at least the treatment of restriction enzyme on the cultured cells.
6/9 Thursday
All wet lab members
10 a.m. - 6:30 p.m. @ KIB
By yesterday, we had prepared for the extraction of plasmids from the transformed cells to examine if the transformation of the biobrick plasmids occurred properly. We took up the required biobricks, transformed them to the competent cells, selected and cultured the transformed cells on the antibiotic-containing solid media, and prepared for the mini-prep experiment, i.e., we had transferred the selected cells, which seemed to contain the biobrick plasmids, into the antibiotic-containing liquid media and cultured them in the incubator. So, we were ready to perform the mini-prep experiment to extract the plasmids from the cultured cells to verify the proper transformation of the biobrick plasmids.
Today, we have performed the mini-prep experiment with the cultured cells. First, we used buffer S1, S2, and S3, PW buffer, and Elution Buffer to extract the cell material from the transformed the cells as well as remove all the molecules except the plasmids. Next, we treated the extracted plasmids with the restriction enzyme solution to check the length of the biobrick part of the plasmids, thereby, confirming the proper transformation. So, we’re ready to measure the length of the biobrick part of each plasmid by DNA gel electrophoresis (Protocol attached).
Tomorrow, we’re going to perform DNA gel electrophoresis to check the length of the DNA pieces produced by the restriction enzyme treatment.
6/10 Friday
All wet lab members
10 a.m. - 6 p.m. @ KIB
By yesterday, we had prepared for checking if the transformation of the biobrick plasmids is achieved properly. We took up the required biobricks, transformed them to the competent cells, selected and cultured the transformed cells, performed mini-prep experiments, and cut the extracted plasmids by treating them with the restriction enzymes. So, we were ready to perform the gel electrophoresis to check the length of the biobrick parts in the plasmids that had been extracted.
Today, we’ve done three things. First, we discovered a new biobrick that is called Inverter that seems useful and tried to amplify it by transforming it into the competent cells and culturing the transformed cells. By the way, the transformation wasn’t achieved properly and we concluded the competent cells that we had made don’t work well and decided to make the competent cells again. Second, we performed the gel electrophoresis with the biobrick parts that had been cut out of the plasmids by the restriction enzymes (protocol attached). By the way, the results were not good – no expected sizes of DNAs, which correspond to the biobrick parts, appear (results summary attached). So finally, we prepared for another mini-prep experiment, deciding to perform the mini-prep again. From the master plate, we picked up the colony of the cells that had been transformed with the biobrick plasmids, transferred them into the liquid media, and cultured them in the incubator.
Tomorrow, we’re going to continue the preparation for another mini-prep and may start the mini-prep.
 "
"