Team:Cambridge/Experiments/Reflectin Thin Films III
From 2011.igem.org
Contents |
Reflectin Thin Films III
Here we set out to assess the quality of thin films produced via the various protein purification protocols namely:
- Ethanol precipitation
- Acetone precipitation
- Wilson inclusion body prep protocol
- Establish that observed iridescence on films are due to reflectin
- Establish the origin of crystallisation - is it due to reflectin or due to impurities
Methods
The films were produced by diluting the pellets from vacuum centrifuge in 120μl of HFIP and centrifuged for 10 mins at 13,000 rpm in a microcentrifuge. The resulting solution was spun at 3000rpm for 30s. Two films were produced for each pellet, the first was not heat-treated after spin-coating the second was heated on a hot plate at 80oc for 2-5min.
Five controls were used:
- GFP - Green Fluorescent Protein was used to assess whether other proteins with unusual amino acid composition was significant
- Ethanol Precipitation Buffer - Used to assess whether impurities due to the reagents used in purification contributed significantly to the production of thin films.
- Acetone Precipitation Buffer- Used to assess whether impurities due to the reagents used in purification contributed significantly to the production of thin films.
- Wilson Protocol Buffer - Used to assess whether impurities due to the reagents used in this quick and dirty inclusion body prep contributed significantly to the production of thin films.
- 10mM Tris & 0.1% Triton X-100 solution - Used to assess whether these reagents used in the final vacuum centrifuge contributed significantly to the production of thin films.
Three types of protein from the various protocols were used:
- Wilson Protocol
- Ethanol Precipitation
- Acetone Precipitation
Observations
Controls
Tris-triton did dissolve in HFIP however does not form a thin film after spin-coating instead a highly regularly-spaced sediment of circular tris solids was deposited on the silicon substrate. On closer inspection these circular solids are surrounded by a ring of iridescence. This showed that tris does not give rise for the iridescence observed in the thin films.
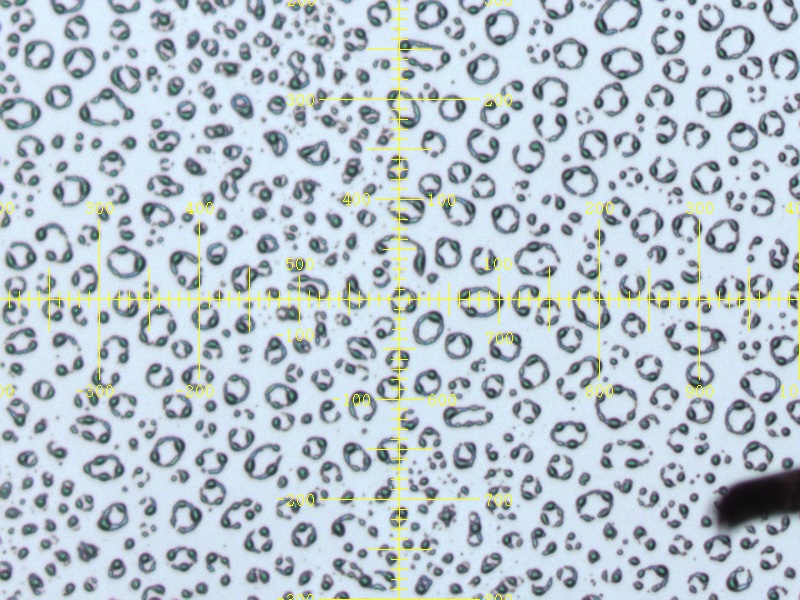
The majority of the dried Ethanol Precipitation Buffer and the Acetone Precipitation Buffer was not soluble in HFIP and a large pellet was seen after centrifugation. What was soluble however formed dense crystalline structures after spin-coating with randomly oriented grain boundaries on the silicon substrate which redissolved with water.
With the Wilson protocol not only did it not dissolve well in HFIP but also no crystallization occurred. Instead any impurities were deposited on the silicon substrate after spin-coating.
Green Fluorescent Protein was found insoluble in HFIP, a mass of clear crystals were left after centrifugation. The protein was found to be denatured after suspension in HFIP as it had lost its original fluorescent green colouration. The resulting spin coat showed deposition of what GFP still remained in the supernatent.
Protein
Spin Coating
Initially the films produced from the same treatment as the buffers above showed vibrant iridescence however the films rapidly crystallised over, adopting a dull sheen reminiscent of the colour of the silicon substrate.
It was found the best films were produced from ethanol and acetone precipitation with virtually no film observed from the wilson protocol the best being isolated patches of iridescence amidst surrounding impurities.
Comparing the films produced with their respective buffers, it can be noted that the crystallization pattern was virtually identical. In fact, one can almost say that whilst reflectin gives the films its iridescence, the crystallization structure is wholly due the impurity present in the buffer which is believed to be urea crystals.
The basis for our conjecture that the crystallisation is due to urea is that this chemical was present in all of the buffers used for during the purification process and was not dialysed out of the eluted protein. Instead the proteins were concentrated via precipitation and subsequently pelleted by centrifugation.
To test this further a quick drop cast was performed with 8M urea used in the extraction buffers on glass microscope slides and imaged under an optical microscope. The result was highly conclusive, the crystalline structure formed was identical to that present in our thin films and further the crystals were colourless!
Flow Coating
In the literature films were cast via flow/blade coating which we have thus far not explored widely due to the lack of suitable equipment. The blade coater in the nanophotonics lab does not allow for adjustment for angles nor changing of blades. Instead we simply explored the limits of blade coating with a normal razor blade. It was found that this was the best method for producing gradient colour ranging across the spectrum however being done by hand control was limited.
Summary
From the results of the experiments above the following points can be noted:
- Reflectin is responsible for the iridescence observed in the thin films and can coat structures like crystals.
- In this batch of proteins, urea is observed to be the culprit for crystallization and dominates the structure of the thin films however the crystalline structure is different to that observed in thin films II which remains a mystery.
- The urea crystals redissolve on application of water which subsequently changes the distribution of the reflectin in the thin films thus 'destroying' it.
- The iridescence properties of reflectin are unique to it, other proteins with weird amino acid content like GFP do not form such thin films nor do proteins like BSA.
We hypothesize that the quality of the films being produced could be improved by either dialysing out the urea or to use alternative reagents like guanidine hydrochloride instead in the purification buffers.
Health and Safety
- HFIP is a powerful polar solvent which is harmful if inhaled or on skin contact therefore all work was carried out under the fume cupboard whilst wearing nitrile gloves, safety goggles and lab coat.
Back to Experiments
 "
"