Team:Washington/Alkanes/Methods
From 2011.igem.org
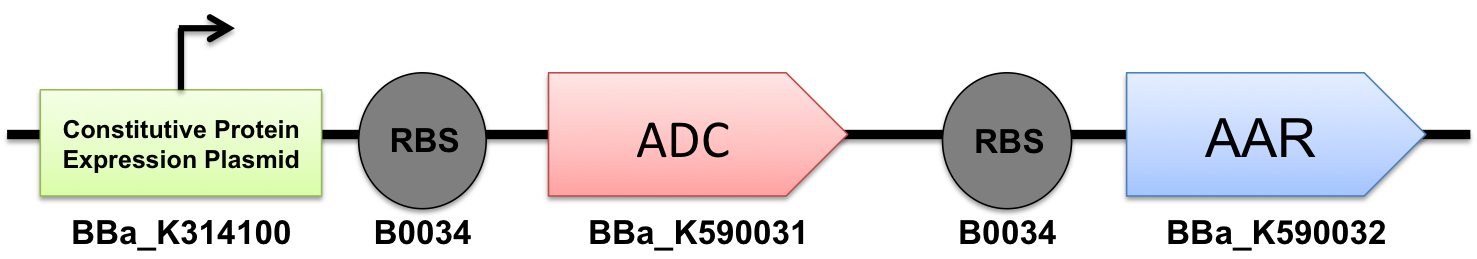
Introducing the PetroBrick
In order to produce alkanes, we need both [http://partsregistry.org/wiki/index.php?title=Part:BBa_K590032 Acyl-ACP Reductase] (AAR) and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K590031 Aldehyde Decarbonylase] (ADC) to work together in the cell. In order to achieve this goal, we used standard cloning methods combining both to construct the [http://partsregistry.org/Part:BBa_K590025 BBa_K590025] Biobrick that contained both AAR and ADC under a high constitutive promoter, each with its own Elowitz standard RBS. This construct successfully synthesized our target product, and thus we have created a new modular alkane-producing platform:
the PetroBrick.
Alkane Production & Extraction
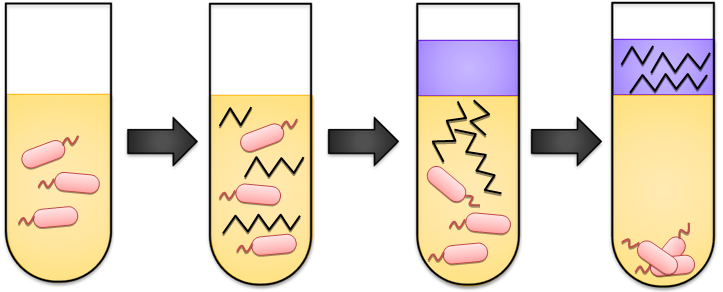
After we had the complete gene assembly in our hands, the next step was to transform it into cells and start them growing for alkane production. We let them grow in 37 degree shaker for 48-72 hours, in sealed glass tubes. After the cells have gone through the alkane production process, the next step is to extract the alkanes out of the cell broth. We add acyl acetate directly into the glass test tube for cell growth. Then we vortex until to everything is well mixed, to make sure all of the alkanes go directly into the ethyl acetate solvent. Next, we spin down the mixture by using a centrifuge at full speed to form three layers (cell pellet, media, and ethyl acetate supernatant). We use only the ethyl acetate layer to send for GCMS analysis. For a more extensive coverage of our media, growth, and extraction techniques, refer to our Protocol page.
Alkane Detection
Gas Chromatography and Mass Spectrometry
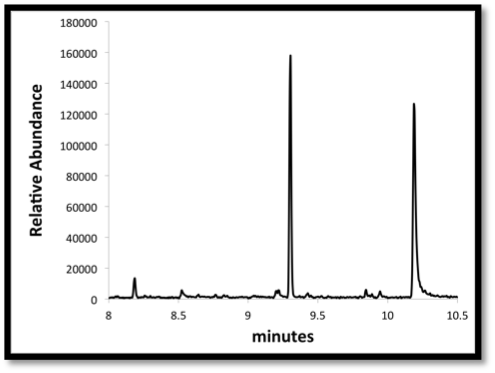
We utilized a Gas Chromatograph / Mass Spectrometer (GCMS) to analyze alkane production concentrations. The GCMS is considered a "specific" test, because it identifies compounds specifically, not just to a category of compounds. It works by separating the individual components of a sample through a capillary column based mainly on its boiling point, similar to fractional distillation. The separated compounds generally elute from the column at different retention times, and are passed to the mass spectrometer.
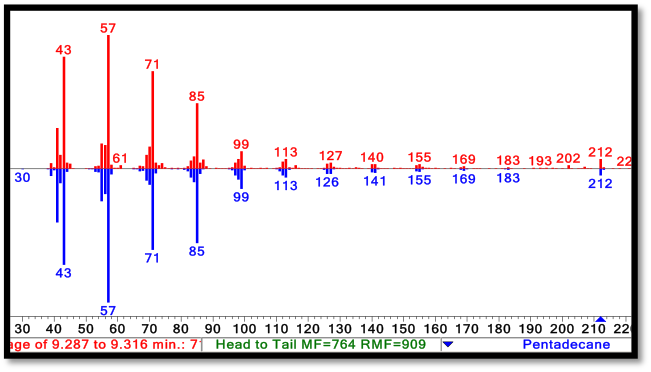
Inside the mass spectrometer each compound is then broken down into its individual molecular components through electron beam ionization. These ions differ in mass-to-charge (m/z) ratios, creating a unique ion de-composition profile for each compound that can be used to identify it through comparison to known chemical standards. Because compounds occasionally have similar elution times or mass spec fingerprints, the combination of analyses results in reducing the chances for overlap.
Gas chromatography: A method used to separate molecules from the media extraction based on boiling point. In this image the temperature is increasing over time, and molecules with a higher boiling point are being eluted and detected by a mass spectrometer. The ion abundance is concentration dependent and can be converted if using a standard curve |
Mass Spectrometry: Molecules exiting gas chromatography enter an electron impact mass spectrometer. The molecules are ionized and fragmented. The resulting spectra is compared to a database of molecules in order to predict its chemical identity. On the top (red) is an experimental spectra of our biologically produced C15 alkane, on the bottom (blue) is the NIST standard spectra for a C15 alkane. The fragmentation pattern and parent ion (blue arrow, bottom right) match perfectly. |
 "
"