This page will provide an overview of the issues related to biological safety and security in the “Track ‘n Trace” project. The biosafety and biosecurity issues involved with the main project are posted on the main safety page.
1. Biological safety identification
Would the materials used in your project and/or your final product pose risks to the safety and health of team members or others in the lab?
The filamentous and spore-forming fungus Aspergillus nidulans (strain ΔpyrG89 ΔpabaB22 nkuATargB ΔriboB2, ordered from [http://www.fgsc.net/ FGSC]; A1147) potentially has an easy route of infection. Nonetheless, according to Kim et al., disease caused by the micro-organism is rarely seen in healthy persons (1997 Jun; jump to references). Correspondingly, the Dutch ‘Regeling Genetisch Gemodificeerde Organismen' (Regulation of Genetically Modified Organisms) classified A. nidulans as Biosafety level (BSL) 1 and allows genetic modifications when these are performed in a ML-II lab.
Project developments may require work with Aspergillus niger in addition to A. nidulans. This micro-organism is also classified as BSL 1 and is therefore subject to the same safety considerations as A. nidulans. During the construction of the BioBrick system, Hygromycin B resistance was used as a selection marker. While this chemical compound is toxic to humans, masks, gloves a lab coat and glasses were worn in preparation of the media. The Track ‘n Trace project involves modifying the leucine metabolism of A. nidulans and using metabolic intermediates to regulate the expression of fluorescent proteins. We do not expect these modifications to increase the pathogenicity or fitness of the A. nidulans strain. Therefore, we do not anticipate any additional safety hazards going beyond those inherent to working with unmodified A. nidulans in a laboratory environment.
Would the materials used in your project and/or your final product pose risks to environmental quality if released by design or accident?
There are several scenarios in which unintentional release of genetically modified material could take place. Labeling of the lab equipment and glassware used is necessary to prevent loss and improper waste disposal by a fellow researcher. The air filtering system will have a hard time in keeping aerosols in the lab when a window gets broken. If this happens through a thunderstorm, an electricity break-down is not unlikely and would increase the chance of release furthermore. Under Good Microbial Practice, though, the formation of aerosols is prevented as much as possible, thus the combination of these probabilities leaves a total probability that is not that high. Direct actions should be taken if it is discovered that an autoclave has been malfunctioning after the waste is discarded outside of the lab. To reduce the risk on environmental contamination it is necessary to check upon the autoclave’s functionality (by monitoring its operational temperature). If the hazards mentioned in this paragraph do happen, it should be reported to the Minister of ‘Housing Spatial Planning and the Environment’ and involved institutions to make the hazards undone as soon and well as possible. The main risk in this project is the accidental release of genetically modified spores into the environment. This could potentially occur within the lab and during transportation if samples are not properly contained and within the lab. Because the life cycle of Aspergillus contains a sporulation phase, it is subject to a higher risk of being released into the environment than organisms that can only replicate through cell division. To reduce the probability of release by sporulation, samples are covered up in petri dishes as much as possible. Also the petri dishes are held upside down when the fungus is transferred, so if spores come free they are mainly directed towards the lab bench. If spores still are released filtration of the air in the ML-II lab would decrease the chance of environmental contamination. The Track ‘n Trace system has a poor survivability in the environment, because it is an auxotroph (leucine is an essential nutrient for the A. nidulans strain).
Risks and benefits
Under Good Microbiological Practices the risk of working with the BioBrick system is rather small to the worker. The Track ‘n Trace project seeks mainly fundamental knowledge, and is an attempt to introduce a new, biotechnologically useful eukaryotic model to iGEM. A better control of intercellular particle transfer in filamentous fungi could also prove beneficial for engineering solid state fermentation cultures though. From this our team has concluded that the potential benefits of executing our project should be carried out to improve knowledge, as the risks are low enough.
2. Biosecurity
Would the materials used in your project and/or your final product pose risks to security through malicious misuse by individuals, groups or states?
On itself, the cell chassis that is used is not considered to be dangerous to human beings. Despite the involved BioBrick systems of our project, the chassis is not pathogenic or harm full. The BioBrick system is dedicated to help in mainly fundamental research. If a person or organization with really bad intentions needs a living toxin producing system, our BioBrick systems would be very inefficient. The biological terrorist (organisation) would have wanted all cells of the fungus producing a toxin. In extend, it would be useless if the cells would make it one by one –in the prolongation of a hypha. Because of the above reasons we think the BioBrick system is reasonably secure.
3. Notification
Although we argued no significant health risks apply to our BioBrick system, it might be found differently by a new user. When it would turn out there is an increased health issue by using the Track ‘n Trace BioBrick part in A. nidulans, the Main page and Experience page (User Reviews) in the Registry of Standard Biological Parts’ catalog would have to be revised. These pages enable modifications, so the users that experienced the unforeseen hazards would be able to add the additional biosafety comments.
4. Biosafety regulation
Does your country have national biosafety regulations or guidelines?
In the Netherlands there are regulations and guidelines to control the production of genetically modified organisms. They are described in detail on the main safety page.
What are the institution's own biosafety rules?
Wageningen UR has its own biosafety rules next to the national biosafety regulations. Its rules can be found on this [http://www.wageningenuniversity.nl/UK/informationfor/Current+students/Student+information/healthsafety/Laboratory+general/?wbc_purpose=basic#basic laboratory safety page].
Did you receive any biosafety and/or lab training before beginning your project?
Next to the biosafety rules from Wageningen UR, techniques for working aseptically to perform Good Microbial Practices are generally learned in a Microbiology introductory course. A lab manager, also called a practical tutor, has introduced every team member to the lab by giving them a “safety tour”. Therein, we came across a lot of, rather general, rules we should take into account when working in the laboratory. The rules deal with: properly discarding biological and chemical waste, the disinfection of the lab bench, the clothing to wear inside the lab, your way of acting during an emergency and more.
Does your institution have an Institutional Biosafety Committee or equivalent group?
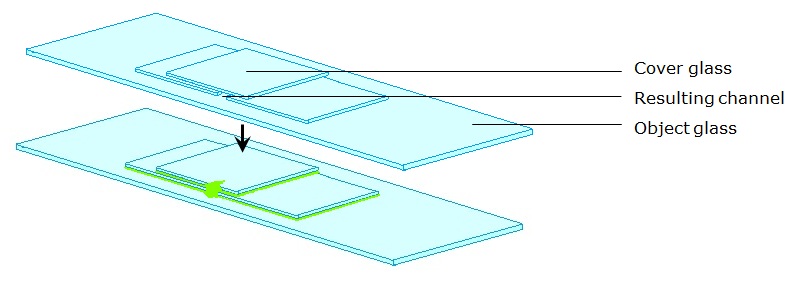
Instead of having a Biosafety Board at Wageningen UR, all chair groups of the institution have their own Biosafety officer(s) (Biologische veiligheidsfunctionaris[sen] in Dutch). Besides Biosafety officers, other safety supervisors are hosted by Wageningen UR. Practical tutors, or lab managers, take an eye on the lab work. Such a person has given the team advice for maintaining biosafety in transportation. In order to study A. nidulans constructs with (confocal) microscopy it would be necessary to transfer the fungus from one building to an other building close by. To do this, he reckoned using a sterile box container sealed with protective film would decrease the probability of release into the environment enough. At the actual point of the microscopy, the sample would have to be exposed in a lab without physical containment. The probability of genetically modified material being released should thus be prevented differently. It was advised by the practical tutor to remove the spore forming bodies from the fungus –to leave the hyphae behind– and seal the openings of the sample with nail polish (as illustrated in the figure below). Another option he gave was to sterilize the surface, by passing it quickly (to prevent damage to the hyphae inside) through a Bunsen flame in the ML-II lab before transport. This project actually was abandoned before these measurements had to be performed (as explained in ‘End of the project’).
Safety improvement suggestions
Some suggestions on how safety could be improved in the iGEM competition and safety of BioBrick systems/parts could be improved are noted on the main safety page
References
Kim M, Shin JH, Suh SP, Ryang DW, Park CS, Kim C, Kook H, Kim J. Aspergillus nidulans infection in a patient with chronic granulomatous disease.
J Korean Med Sci. 1997 Jun;12(3):244-248. URL: http://synapse.koreamed.org/DOIx.php?id=10.3346%2Fjkms.1997.12.3.244. This is an unofficial translation of the article. The publisher has not endorsed this translation. Copyright © 1997 The Korean Academy of Medical Sciences. The article is published under the [http://creativecommons.org/licenses/by-nc/3.0/ Attribution-NonCommercial 3.0 Unported Creative Commons] license.
 "
"