Team:Wageningen UR/Project/Devices
From 2011.igem.org
Matthijnwur (Talk | contribs) (→Custom fluidic device designed by Team Wageningen UR to measure oscillations) |
Matthijnwur (Talk | contribs) (→Custom fluidic device designed by Team Wageningen UR to measure oscillations) |
||
| Line 34: | Line 34: | ||
'''Bacterial growing platforms''' | '''Bacterial growing platforms''' | ||
| - | This flow device can accommodate two bacterial growing platforms, a micro-sieve and a micro-dish. The micro-sieve as depicted is figure | + | |
| + | This flow device can accommodate two bacterial growing platforms, a micro-sieve and a micro-dish. The micro-sieve as depicted is figure 2 are membranes which have evenly distributed pores of 200 nm in diameter. These membranes are used in dairy industry to sterilise dairy products by removing micro-organism through filtration. Through filtration a cake of cells will form on the membrane, potentially giving us a platform capable of inducing and giving a mean of monitoring oscillatory behaviour of a population of e. coli cells. | ||
| + | |||
| + | [[File:microsieve.jpg]] | ||
| + | |||
| + | '''Fig.2:''' ''Raster electron microscopy image of the micro-sieve with saccharomyces cerevisiae cells.'' | ||
| + | |||
| + | |||
| + | Another bacterial platform the micro-dish as depicted in figure 3 is a thin acrylic layer with pores which is superimposed on a layer of porous aluminium oxide. The wells seen in figure 3 have a diameter of 180 um and a depth of 40 um. Because the bottom of the wells are porous, nutrients can freely diffuse through the material to any cells contained in the well, these cells will divide until they are physically constraint by the borders of the well. Potentially giving us another platform capable of inducing and monitoring oscillatory behaviour of a population of e.coli cells. | ||
| + | |||
| + | |||
| + | [[File:microdish.jpg]] | ||
| + | |||
| + | |||
| + | '''Fig.3:''' ''Light microscopy image of the micro dish.'' | ||
| + | |||
| + | '''Implementation of both bacterial platforms in the design of the flow device.''' | ||
| + | |||
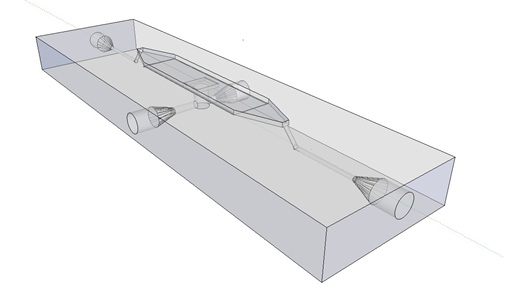
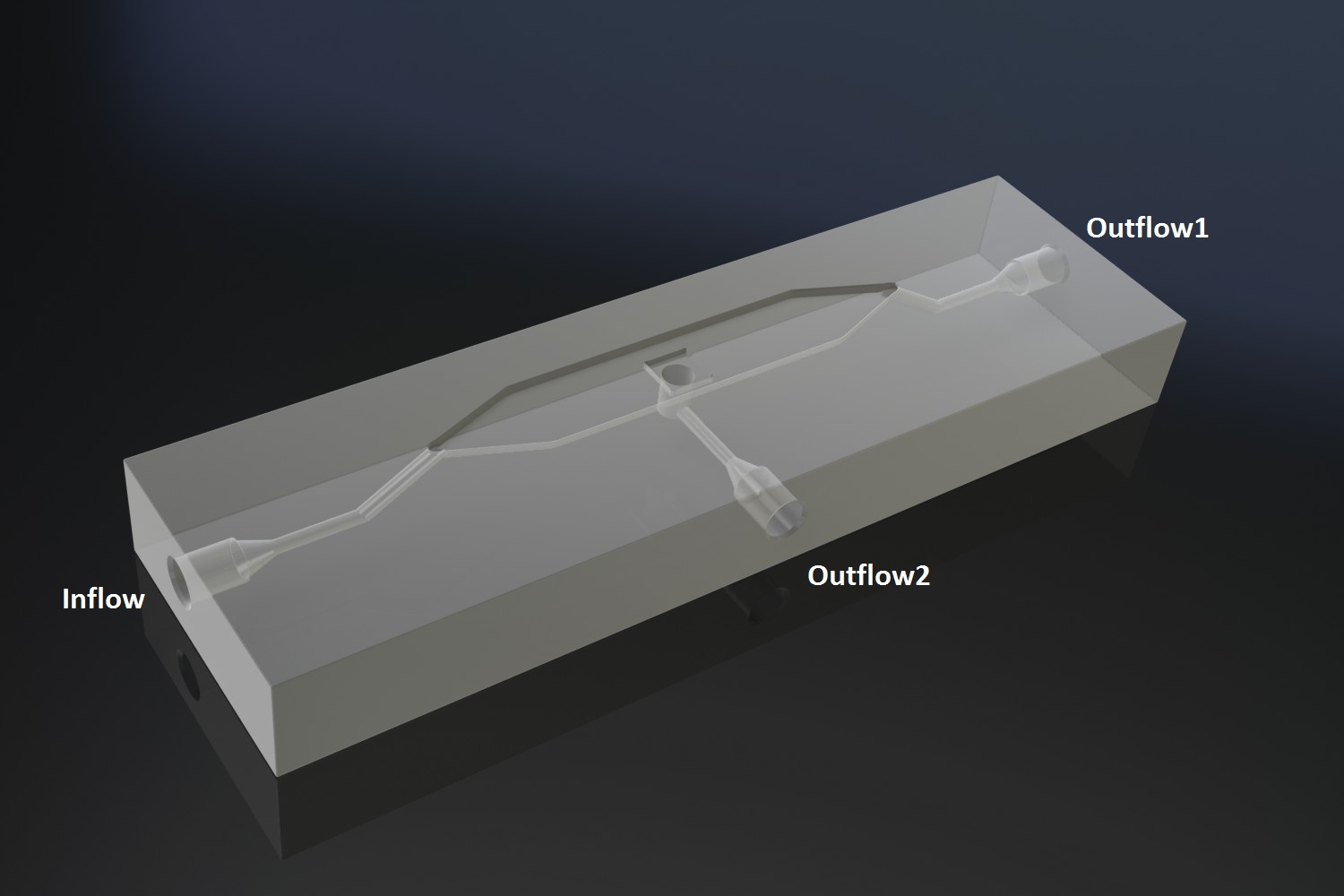
| + | Potentially inducing and observing oscillatory behaviour in a population of e.coli cells with the means of using a micro-sieve, a cake of cells should be formed on the membrane. In dairy industry they do this by applying the filtrate through an inflow port leading to a chamber, which houses the micro-sieve. Because an overpressure is produced in the chamber liquid will be forced through the sieve, and the suspended particles in our case e.coli cells will aggregate on the sieve. However to prevent that the pressure in the chamber reaches a too high pressure that cells get lysed by being pushed through the filter also an outflow port is included. In the figure below the functional components of the flow device are depicted. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
Revision as of 19:45, 19 September 2011
 "
"