Team:Brown-Stanford/REGObricks/Biocementation
From 2011.igem.org
Ho.julius.a (Talk | contribs) (→Introduction) |
Maxsong123 (Talk | contribs) (→Biocementation) |
||
| Line 2: | Line 2: | ||
== '''Biocementation''' == | == '''Biocementation''' == | ||
| - | + | ||
| + | === '''What is Biocementation?''' === | ||
| + | |||
| + | |||
| + | “Biocementation” is the growing field of employing biological agents as catalysts for increasing the stiffness and strength of soil [1]. The particle branch of biocementation that we investigated in our project this summer was “Microbial Induced Carbonate Precipitation (MICP)”, a process by which microorganisms metabolize various nitrogen products to increase ambient pH. The salient organism in our case is Sporosarcina pasteurii (previously Bacillus pasteurii), which utilizes the hydrolysis of urea to generate carbon dioxide and ammonia, which ultimately convert to carbonate ions in a basic environment. Carbonate fuses with available metals in solution to precipitate in crystal formation, and fuse surrounding particles together. | ||
| + | |||
| + | === '''The Chemical Reaction''' === | ||
| + | |||
| + | Urease (urea amidohydrolase; EC 3.5.1.5) is a six-subunit protein assembled from three alpha and three beta subunits. | ||
| + | Contained in the center of the urease enzyme is dual nickel metallocore, crucial to its activity (Mobley HL et al). Studies varying the concentration of nickel show a strong dependence on the availability of metal anions; mutations that remove the expression of an accessory metal-transport protein leads to dramatic decreases in urease activity []. During each cycle of activity, urease breaks down one urea molecule into one molecule of ammonia and one molecule of carbamate, which is unstable and decomposes to another molecule of ammonia and bicarbonate. Each of the ammonia molecules have a pKa of 9.31, and take up protons from the cytoplasm. They are then shuttled out of the cell to create an proton gradient for ATP-generation. Outside the cell, ammonium contributes to an increase of pH in the surrounding environment. The cell itself also serves as a nucleation site, as positively charged calciums in solution are attracted by its negative membrane. The gradual evolution of carbonate from the basic environment begins the process of calcium precipitation, and the rest, they say, can be seen in stone! | ||
| + | |||
| + | [[File:Brown-Stanford-ureasereaction.JPG|300px|frame|Evolution of two ammonium ions and one carbonate ion from the hydrolysis of urea/]] | ||
| + | |||
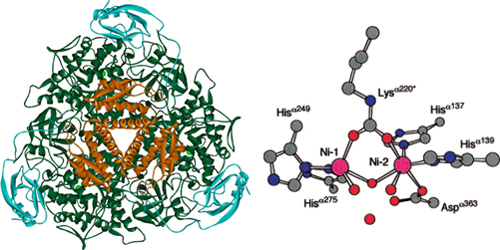
| + | [[File:Brown-Stanford-ureaseactive.JPG|300px|frame|Urease Active Site, Credit to http://bioinorg.agrsci.unibo.it/]] | ||
| + | |||
| + | The genetic sequence of the urease has been ascertained for several strains of bacteria, including H. pylori, and some partial mappings have been done for individual subcomponents of S. pasteurii [] []. There have also been occasional mentions of strains of recombinant bacteria containing urease function [] [] [] though unfortunately, none currently in a biobricked form. While the study of urease function in H. pylori has matured under medicinal interest over the years, the genetic expression of urease is still a field of ongoing research. Ambitiously, we decided that one of the project goals for REGObricks would be to curate an modular, enzymatically functional urease to the iGEM registry of Standardized Parts. | ||
| + | (link here) __________________________________ | ||
| + | |||
| + | |||
{{:Team:Brown-Stanford/Templates/Foot}} | {{:Team:Brown-Stanford/Templates/Foot}} | ||
Revision as of 05:51, 26 September 2011
Biocementation
What is Biocementation?
“Biocementation” is the growing field of employing biological agents as catalysts for increasing the stiffness and strength of soil [1]. The particle branch of biocementation that we investigated in our project this summer was “Microbial Induced Carbonate Precipitation (MICP)”, a process by which microorganisms metabolize various nitrogen products to increase ambient pH. The salient organism in our case is Sporosarcina pasteurii (previously Bacillus pasteurii), which utilizes the hydrolysis of urea to generate carbon dioxide and ammonia, which ultimately convert to carbonate ions in a basic environment. Carbonate fuses with available metals in solution to precipitate in crystal formation, and fuse surrounding particles together.
The Chemical Reaction
Urease (urea amidohydrolase; EC 3.5.1.5) is a six-subunit protein assembled from three alpha and three beta subunits.
Contained in the center of the urease enzyme is dual nickel metallocore, crucial to its activity (Mobley HL et al). Studies varying the concentration of nickel show a strong dependence on the availability of metal anions; mutations that remove the expression of an accessory metal-transport protein leads to dramatic decreases in urease activity []. During each cycle of activity, urease breaks down one urea molecule into one molecule of ammonia and one molecule of carbamate, which is unstable and decomposes to another molecule of ammonia and bicarbonate. Each of the ammonia molecules have a pKa of 9.31, and take up protons from the cytoplasm. They are then shuttled out of the cell to create an proton gradient for ATP-generation. Outside the cell, ammonium contributes to an increase of pH in the surrounding environment. The cell itself also serves as a nucleation site, as positively charged calciums in solution are attracted by its negative membrane. The gradual evolution of carbonate from the basic environment begins the process of calcium precipitation, and the rest, they say, can be seen in stone!
The genetic sequence of the urease has been ascertained for several strains of bacteria, including H. pylori, and some partial mappings have been done for individual subcomponents of S. pasteurii [] []. There have also been occasional mentions of strains of recombinant bacteria containing urease function [] [] [] though unfortunately, none currently in a biobricked form. While the study of urease function in H. pylori has matured under medicinal interest over the years, the genetic expression of urease is still a field of ongoing research. Ambitiously, we decided that one of the project goals for REGObricks would be to curate an modular, enzymatically functional urease to the iGEM registry of Standardized Parts. (link here) __________________________________
 "
"