Team:Harvard/Template:NotebookDataJuly2
From 2011.igem.org
(Difference between revisions)
(Created page with "<div id="707" style="display:none"> ==July 7th== ===Team ZF Assembly=== Our primers still haven't arrived. We are working with the Web Design Team. ===Team Wolfe Selection=== ...") |
(→Team ZF) |
||
| (12 intermediate revisions not shown) | |||
| Line 20: | Line 20: | ||
**samples prepared for sequencing tomorrow | **samples prepared for sequencing tomorrow | ||
| - | [[File: | + | [[File: HARV2011.07.07.hisBseq labeled.png|thumb|none|MAGE 2 samples for sequencing 7/7/11]] |
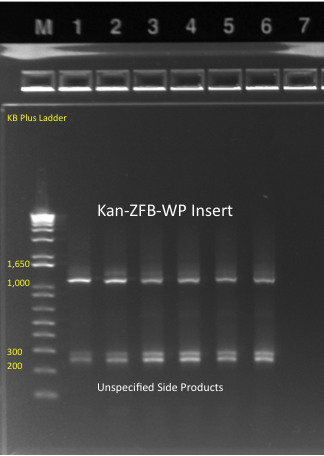
*Kan-ZFB insert check PCR results: | *Kan-ZFB insert check PCR results: | ||
| Line 26: | Line 26: | ||
**Results: some of the reactions, strangely, did not work, but the ones that did showed a short band and thus did not have the insert (see below) | **Results: some of the reactions, strangely, did not work, but the ones that did showed a short band and thus did not have the insert (see below) | ||
| - | [[File: | + | [[File: HARV2011.07.07,kanZFBinsert_check1 labeled.png|thumb|none|1529620 locus PCR 7/7/11]] |
| - | [[File: | + | [[File: HARV2011.07.07,kanZFBinsert_check2 labeled.png|thumb|none|1529620 locus PCR 7/7/11]] |
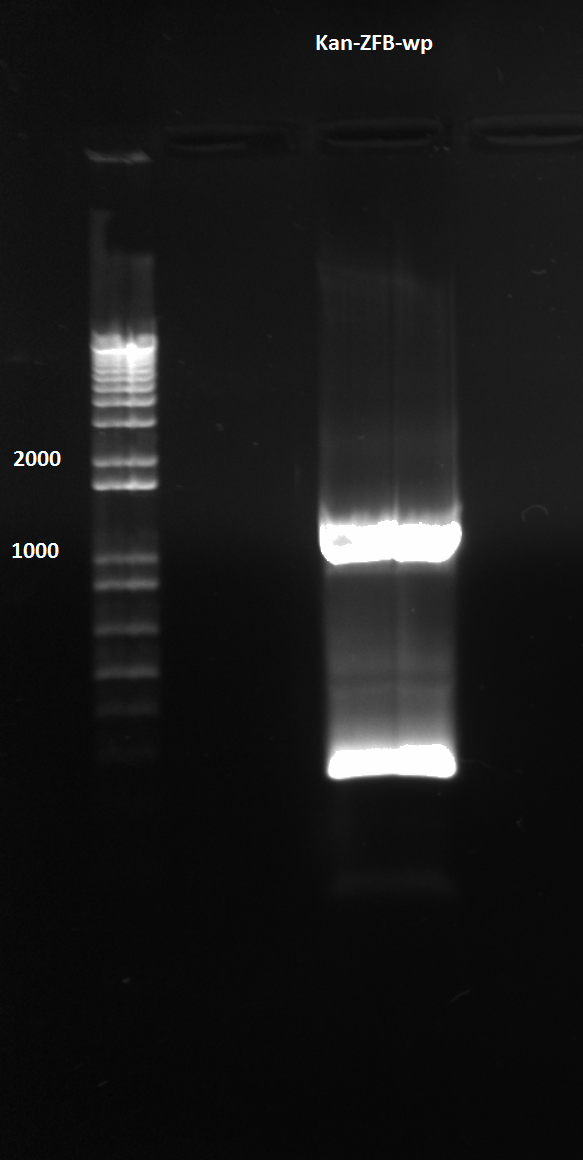
*HisBNuke3 MAGE round 3: | *HisBNuke3 MAGE round 3: | ||
| Line 49: | Line 49: | ||
| - | [[File: | + | [[File:HARVKAN-ZFB-WP.png|200px|thumb|none|Kan-ZFB-WP]] |
===Web Design=== | ===Web Design=== | ||
| Line 57: | Line 57: | ||
*Slideshow on front page | *Slideshow on front page | ||
**First slide: Brief text project description, brief youtube video description (in layman's terms) | **First slide: Brief text project description, brief youtube video description (in layman's terms) | ||
| - | + | </div> | |
<div id="708" style="display:none"> | <div id="708" style="display:none"> | ||
| + | |||
==July 8th== | ==July 8th== | ||
| Line 77: | Line 78: | ||
====Gel purification of PCR product==== | ====Gel purification of PCR product==== | ||
We had to gel purify our PCR product in preparation for isothermal assembly, using [[Protocols#Gel_purification| our protocol for gel purification]]. The result from running the gel can be seen below - we cut out the bands at 709 bp, the expected size of our product. | We had to gel purify our PCR product in preparation for isothermal assembly, using [[Protocols#Gel_purification| our protocol for gel purification]]. The result from running the gel can be seen below - we cut out the bands at 709 bp, the expected size of our product. | ||
| - | [[Image: | + | [[Image:HARV2011.07.08_omega_+_zif268_with_overhangs_annotated.png]] |
In the final step of the purification process, we eluted our DNA in buffer EB. We used the nanodrop spectophotometer to determine the concentration of DNA present. | In the final step of the purification process, we eluted our DNA in buffer EB. We used the nanodrop spectophotometer to determine the concentration of DNA present. | ||
| Line 98: | Line 99: | ||
*Results: PCR only produced a smaller side product. This is strange since we also ran a gel of all our different kan-ZFB-hisura purifications and they all run at the correct size (see images below). | *Results: PCR only produced a smaller side product. This is strange since we also ran a gel of all our different kan-ZFB-hisura purifications and they all run at the correct size (see images below). | ||
| - | [[File: | + | [[File: HARV2011.07.08.ECnr2MAGEround2-21to24delrpoZ&thioKAN-ZFB labeled.png|thumb|none|Thio kan-ZFB 7/8/11]] |
| - | [[File: | + | [[File: HARV2011.07.08.kanzfbDNA(labeled).png|thumb|none|kan-ZFB insert purifications]] |
===Team TolC=== | ===Team TolC=== | ||
| Line 111: | Line 112: | ||
===Team Web Design=== | ===Team Web Design=== | ||
*Edited/Re-drafted our project description, see Dropbox (It's a bit long as is now, but the excess informaiton that we cut out can still probably be used somewhere on our website | *Edited/Re-drafted our project description, see Dropbox (It's a bit long as is now, but the excess informaiton that we cut out can still probably be used somewhere on our website | ||
| - | *Sketched draft pages of the public wiki | + | *Sketched draft pages of the public wiki |
| - | **Homepage- first draft sketch done based on the characteristics and requirements we'd discussed. However, there are a few issues (i.e., how to include some of the iGEM wiki reuqirements) | + | **Homepage- first draft sketch done based on the characteristics and requirements we'd discussed. However, there are a few issues (i.e., how to include some of the iGEM wiki reuqirements).</div> |
<div id="709" style="display:none"> | <div id="709" style="display:none"> | ||
| + | |||
==July 9== | ==July 9== | ||
===Team ZF=== | ===Team ZF=== | ||
| Line 121: | Line 123: | ||
*The concentration of the w+zif268 was 54.3 ng/ul. | *The concentration of the w+zif268 was 54.3 ng/ul. | ||
| - | Using [ | + | Using [https://2011.igem.org/Team:Harvard/Protocols#Isothermal_assembly our isothermal assembly protocol] we determined we needed: |
*0.63 ul backbone (length: ~2.2 kb) | *0.63 ul backbone (length: ~2.2 kb) | ||
*0.59 ul w+zif268 (length: ~0.7 kb) | *0.59 ul w+zif268 (length: ~0.7 kb) | ||
| Line 133: | Line 135: | ||
*500 ul LB | *500 ul LB | ||
| - | We plated the cells, [ | + | We plated the cells, [https://2011.igem.org/Team:Harvard/Protocols#Cultures using this protocol], on spec plates, one of which contained 50 ul of cells and the other 150 ul of cells. The cells have been incubated at 37*, and will be left there overnight. Cells that have taken up the plasmid have spec-resistance and should be able to survive. Tomorrow, we will be picking colonies and performing PCR on them to determine whether they contain w+zif268. |
===Team Wolfe=== | ===Team Wolfe=== | ||
| Line 145: | Line 147: | ||
===Team ZF=== | ===Team ZF=== | ||
====PCR to check for omega+zif268==== | ====PCR to check for omega+zif268==== | ||
| - | All colonies that grew on the plates we left overnight should have spec-resistance, but they may not have the omega and zif268 unit that we inserted | + | All colonies that grew on the plates we left overnight should have spec-resistance, but they may not have the omega and zif268 unit that we inserted yesterday with isothermal assembly. We performed a PCR on 13 colonies picked from the 50 ul plate to find a colony that contains the w+zif268 insertion. We used the same recipe at our PCR reaction [[#PCR of omega+zif268 with overhangs on Friday. If the insert is present, then we should see a band at 709 bp. We also included 2 positive controls: our isothermal assembly product and our selection strain from which the w+zif268 was originally copied. |
We ran an agarose gel to check the bands: | We ran an agarose gel to check the bands: | ||
{| | {| | ||
| - | |[[File: | + | |[[File:HARV2011_07_10_transformation_colony_pcr_lane_1-8.png|thumb|Lanes 1-8]] |
| - | |[[File: | + | |[[File:HARV2011.07.10_transformation_colony_pcr_lane_9-16.png|thumb|Lanes 9-16]] |
|} | |} | ||
| - | None of the colonies displayed any bands, except for primer residues. Lane 16 contained the selection plasmid, on which we performed [ | + | None of the colonies displayed any bands, except for primer residues. Lane 16 contained the selection plasmid, on which we performed [https://2011.igem.org/Team:Harvard/Protocols#PCR_purification a successful PCR] on Friday, so we definitely expected a band at 709 bp. Because the positive controls did not have a band at 709 bp, we suspect that something went wrong with the PCR. We plan on re-running the PCR tomorrow.</div> |
<div id="711" style="display:none"> | <div id="711" style="display:none"> | ||
| + | |||
==July 11th== | ==July 11th== | ||
===Team ZF=== | ===Team ZF=== | ||
| Line 216: | Line 219: | ||
|} | |} | ||
| - | Then we performed a PCR on the 12 miniprep products, the 12 colonies in LB, as well as 3 controls (negative control, isothermal assembly product, selection strain) to find a colony that has the omega+zif26 insertion. | + | Then we performed a PCR on the 12 miniprep products, the 12 colonies in LB, as well as 3 controls (negative control, isothermal assembly product, selection strain) to find a colony that has the omega+zif26 insertion: |
| + | *10 ul KAPA readymix | ||
| + | *0.75 ul of hindIII (primer) | ||
| + | *0.75 ul of wF+plLacO (primer) | ||
| + | *1.2 ul template | ||
| + | *12.3 ul water | ||
| + | |||
| + | The first steps of the PCR involved heating at 95* (for 5 minutes) and 98* (for 20 seconds). Annealing temperature was 56*. Extension time was 30 seconds. | ||
We ran a 1% 100 ml. agarose gel at 120V to check the success of the PCR. | We ran a 1% 100 ml. agarose gel at 120V to check the success of the PCR. | ||
{| | {| | ||
| - | |[[File: | + | |[[File:HARV7.11.11.omegazif_transformed_annotated.png|thumb|Gel of our colonies, miniprep-ed colonies, and controls to check for w+zif268 insertion]] |
|} | |} | ||
| Line 228: | Line 238: | ||
{| | {| | ||
| - | |[[File: | + | |[[File:HARV7.11.11.omegazif_transformed_egel_annotated.png|thumb|Gel to ensure our bands were the correct size (709 bp)]] |
|} | |} | ||
| Line 240: | Line 250: | ||
**KAPA protocol using M13_F and M13_R primers (anneal to the vector), 50˚C annealing, 2min elongation | **KAPA protocol using M13_F and M13_R primers (anneal to the vector), 50˚C annealing, 2min elongation | ||
**E gel results: lots of bands but rather messy and not exactly what we'd expect. We'll try to verify the presence of the plasmid in a few other ways. | **E gel results: lots of bands but rather messy and not exactly what we'd expect. We'll try to verify the presence of the plasmid in a few other ways. | ||
| - | [[File: | + | [[File: HARVPSR01 test1(labeled).png|thumb|none|pSR01 colony PCR 7/11/11]] |
*Sequencing: | *Sequencing: | ||
**We sent out the PCR samples with M13_R to Genewiz | **We sent out the PCR samples with M13_R to Genewiz | ||
| Line 250: | Line 260: | ||
*In case pSR01 does not rescue the selection strain's growth phenotype, we will PCR 96 colonies from the MAGE3 plates and send them out for sequencing tomorrow. | *In case pSR01 does not rescue the selection strain's growth phenotype, we will PCR 96 colonies from the MAGE3 plates and send them out for sequencing tomorrow. | ||
**Used 9µL KAPA, His_F and His_R primers, 3µL suspended colony (out of 7--the remaining 4 grown in LB+amp) | **Used 9µL KAPA, His_F and His_R primers, 3µL suspended colony (out of 7--the remaining 4 grown in LB+amp) | ||
| - | [[File: | + | [[File: HARV2011.07.11.MAGE 3 96 A1-H1.png|thumb|none|MAGE3 colonies for sequencing 7/11/11]] |
'''Thio kanZFB:''' | '''Thio kanZFB:''' | ||
*We retried the PCR to put modified nucleotides on the ends of the kan-ZFB-wp-hisura insert using the same procedure as 7/8/11 | *We retried the PCR to put modified nucleotides on the ends of the kan-ZFB-wp-hisura insert using the same procedure as 7/8/11 | ||
**E gel: reactions didn't work | **E gel: reactions didn't work | ||
| - | [[File: | + | [[File: HARV2011.07.11.thiokanZFB(labeled).png|thumb|none|thio kan-ZFB PCR 7/11/11]] |
*Tried again with a longer elongation time (3 min) and let run overnight. | *Tried again with a longer elongation time (3 min) and let run overnight. | ||
| Line 263: | Line 273: | ||
===Team Web=== | ===Team Web=== | ||
| - | * | + | *Looked at good wikis from last year. </div> |
<div id="712" style="display:none"> | <div id="712" style="display:none"> | ||
| + | |||
==July 12th== | ==July 12th== | ||
===Team ZF=== | ===Team ZF=== | ||
| Line 276: | Line 287: | ||
{| | {| | ||
| - | |[[File: | + | |[[File:HARV2011.7.12_nine_ultramers_product_annotated.png|thumb|Gel of our product from the ultramer PCR. Note that the first lane's PCR was done in a single tube, while all the others were done in a strip.]] |
|} | |} | ||
| Line 282: | Line 293: | ||
{| | {| | ||
| - | |[[File: | + | |[[File:HARV2011.7.12_nine_ultramers_product_try2_annotated.png|thumb|Gel of our product after PCR purification ]] |
|} | |} | ||
| - | Even after the gel purification, our bands were streaky. We decided to redo the PCR, placing all the reactions in single tubes, performing a touchdown PCR instead. Our annealing temperature started at 70*, repeated for 2 cycles, and then decreased 2*, every two cycles, until it reached 58* (the optimal annealing temperature), where it remained until the 25 cycles were complete. We left this PCR going overnight. (See | + | Even after the gel purification, our bands were streaky. We decided to redo the PCR, placing all the reactions in single tubes, performing a touchdown PCR instead. Our annealing temperature started at 70*, repeated for 2 cycles, and then decreased 2*, every two cycles, until it reached 58* (the optimal annealing temperature), where it remained until the 25 cycles were complete. We left this PCR going overnight. (See July 13th for the gel image.) |
====PCR of expression plasmid cross-junction==== | ====PCR of expression plasmid cross-junction==== | ||
| - | We also performed a second PCR on colony nine (the colony we decided with which we decided to proceed) to confirm that our omega+zif268 in the spec backbone was successfully inserted into the ''E. coli''. The expected product was the cross-junction on the plasmid, approximately 1.4 kb. We used the same recipe as that used on | + | We also performed a second PCR on colony nine (the colony we decided with which we decided to proceed) to confirm that our omega+zif268 in the spec backbone was successfully inserted into the ''E. coli''. The expected product was the cross-junction on the plasmid, approximately 1.4 kb. We used the same recipe as that used on July 9th, except with a forward primer of PZE23G-3581 F and a reverse primer of PZE23G-2133 R, and had a 55* annealing temperature and an extension time of 45 seconds. |
After running an e-gel, we confirmed that our w+zif268 was inserted into the plasmid. | After running an e-gel, we confirmed that our w+zif268 was inserted into the plasmid. | ||
{| | {| | ||
| - | |[[File: | + | |[[File:HARV2011.7.12_omega+linker_and_crossjunction_omgzif-spec_plasmid_annotated.png|thumb|'''Lane 3''': Gel of our PCR to check for the cross-junction in colony 9.]] |
|} | |} | ||
| Line 303: | Line 314: | ||
{| | {| | ||
| - | |[[File: | + | |[[File:HARV2011.7.12_omega+linker_and_crossjunction_omgzif-spec_plasmid_annotated.png|thumb|'''Lanes 1 and 2''': Gel of our PCR to get omega subunit.]] |
|} | |} | ||
| Line 312: | Line 323: | ||
{| | {| | ||
| - | |[[File: | + | |[[File:HARV2011.7.12_OZ052_and_OZ123_ultramers_(last_two_lanes)_annotated.png|thumb|'''Last two lanes''': OZ052 and OZ123 products, respectively]] |
|} | |} | ||
| Line 324: | Line 335: | ||
**In case this mutation is correct but lambda red in the selection strain still eludes us, we designed MAGE oligos for use in EcNR2 to either delete the two aa or put 3 stop codons after the HPase region. | **In case this mutation is correct but lambda red in the selection strain still eludes us, we designed MAGE oligos for use in EcNR2 to either delete the two aa or put 3 stop codons after the HPase region. | ||
*culture PCR results: cultures 3-4 had a strong band (and 2 and 5 weak ones) around 850-1000bp. It is interesting how different these results are from yesterday's colony PCR gel. | *culture PCR results: cultures 3-4 had a strong band (and 2 and 5 weak ones) around 850-1000bp. It is interesting how different these results are from yesterday's colony PCR gel. | ||
| - | [[File: | + | [[File: HARV2011.07.12.pSR01 with phospho primers(labeled).png|thumb|none|pSR01 culture and thio kan-ZFB PCRs 7/12/11]] |
*Sequencing results: The sequences sent from Genewiz aligned well with the pSR01 plasmid sequence and covered pretty much all of URA3. This implies that the cells did indeed take up our plasmid. | *Sequencing results: The sequences sent from Genewiz aligned well with the pSR01 plasmid sequence and covered pretty much all of URA3. This implies that the cells did indeed take up our plasmid. | ||
*Growth phenotype: | *Growth phenotype: | ||
| Line 363: | Line 374: | ||
::In case you haven't already, please see Alain's message below: | ::In case you haven't already, please see Alain's message below: | ||
| - | ::Every member of the Harvard iGEM team (unless you are absent on Friday) has to attend the next lab Safey/Biosafety Blood born pathogens training this coming Friday 7/15/2011 from 10 am to 12 noon. The training takes place in the Biological laboratories (16 Divinity Ave) room 1058. If you cannot go and have a very good reason let me (''Alain'') know. If you attended a similar training session on the Cambridge campus or at the Medical school you don't have to attend. Otherwise you have to go.</div> | + | ::Every member of the Harvard iGEM team (unless you are absent on Friday) has to attend the next lab Safey/Biosafety Blood born pathogens training this coming Friday 7/15/2011 from 10 am to 12 noon. The training takes place in the Biological laboratories (16 Divinity Ave) room 1058. If you cannot go and have a very good reason let me (''Alain'') know. If you attended a similar training session on the Cambridge campus or at the Medical school you don't have to attend. Otherwise you have to go.</div><div id="713" style="display:none"> |
| - | <div id="713" style="display:none"> | + | |
==July 13th== | ==July 13th== | ||
===Team ZF=== | ===Team ZF=== | ||
| Line 372: | Line 382: | ||
{| | {| | ||
| - | |[[File: | + | |[[File:HARV2011.7.13_ultramers_annotated.png|thumb|Gel of our ultramer touchdown PCR product]] |
|} | |} | ||
| Line 409: | Line 419: | ||
***KAPA, 1529620-flanking primers, 90 sec elongation and 56 anneal, 25 cycles | ***KAPA, 1529620-flanking primers, 90 sec elongation and 56 anneal, 25 cycles | ||
***Results: 300bp band--the streaks on the plate are bacteria, but they do not have the insert. | ***Results: 300bp band--the streaks on the plate are bacteria, but they do not have the insert. | ||
| - | [[File: | + | [[File: HARV2011.07.13 Lambda red streaky plate(labeled).png|thumb|none|1529620 locus PCR of EcNR2 lambda red 7/13/11]] |
*We also retried the thio kan-ZFB PCR using some new primer aliquots. Once again it failed. | *We also retried the thio kan-ZFB PCR using some new primer aliquots. Once again it failed. | ||
| - | [[File: | + | [[File: HARV2011.07.13.Phospho thio 4(labeled).png|thumb|none|Thio kan-ZFB PCR 7/13/11]] |
*Because this PCR keeps failing, we're going to try a different way and do two separate and shorter PCRs to be joined later in an overlap PCR: | *Because this PCR keeps failing, we're going to try a different way and do two separate and shorter PCRs to be joined later in an overlap PCR: | ||
**PCR of kan cassette: kan cassette as template (diluted 6/16 aliquot), kan F phosphothio and kan R primers, KAPA, 56˚C anneal and 90 sec elongation | **PCR of kan cassette: kan cassette as template (diluted 6/16 aliquot), kan F phosphothio and kan R primers, KAPA, 56˚C anneal and 90 sec elongation | ||
| Line 430: | Line 440: | ||
***10.7 ng/ | ***10.7 ng/ | ||
| - | [[File: | + | [[File: HARV2011.07.14.TolCinsertionconstruct(labeled).png|thumb|none|KAN-ZFB-wp construct with tolC homology 7/13/11]] |
===Team Web Design=== | ===Team Web Design=== | ||
| Line 447: | Line 457: | ||
{| | {| | ||
| - | |[[File: | + | |[[File:HARV2011.7.14_9_ultramer_isotherm_asm_rxn_with_junction_primers_annotated.png|thumb|1.2% E-gel of our ultramer isothermal assembly reaction product]] |
|} | |} | ||
| Line 458: | Line 468: | ||
{| | {| | ||
| - | |[[File: | + | |[[File:HARV2011.7.14_ultramers.pcr1_touchdown_pcr_iso_assm_annotated.png|thumb|Products ran in an agarose gel. Note that wells 78-80 on the left side of the gel are empty due to sample mysteriously floating out of the well.]] |
|} | |} | ||
| Line 470: | Line 480: | ||
'''thio kan and thio ZFB-wp-hisura PCR:''' | '''thio kan and thio ZFB-wp-hisura PCR:''' | ||
*Ran overnight PCR of kan cassette and ZFB-wp-hisura on E gel: looks great! | *Ran overnight PCR of kan cassette and ZFB-wp-hisura on E gel: looks great! | ||
| - | [[File: | + | [[File: HARV2011.07.14.thiokan+thioZFBhisura(labeled).png|thumb|none|thio kan and thio ZFB-wp-hisura PCR 7/14/11]] |
*PCR purification with Qiagen kit: | *PCR purification with Qiagen kit: | ||
**added an additional 300µL buffer PB to adjust the pH | **added an additional 300µL buffer PB to adjust the pH | ||
| Line 480: | Line 490: | ||
**primers (kan f phosphothio and ura3 r phosphothio) added after 10 cycles | **primers (kan f phosphothio and ura3 r phosphothio) added after 10 cycles | ||
**Results: PCR worked but with tons of side products so we will try to optimize the procedure. Expected product = 3Kb, notable side products = 2Kb, 4Kb. N.B. The samples were accidentally loaded while the gel was running so they are staggered on the gel. | **Results: PCR worked but with tons of side products so we will try to optimize the procedure. Expected product = 3Kb, notable side products = 2Kb, 4Kb. N.B. The samples were accidentally loaded while the gel was running so they are staggered on the gel. | ||
| - | [[File: | + | [[File: HARV2011.07.14.thio kanZFBhisura overlap(labeled).png|thumb|none|thio kan-ZFB-wp-hisura overlap PCR (with loading error)]] |
*Gel stab PCR | *Gel stab PCR | ||
** DNA from the 3Kb e-gel band were removed using the stabbing technique and placed in 4 respective reaction tubes (diluted in 20µL water) | ** DNA from the 3Kb e-gel band were removed using the stabbing technique and placed in 4 respective reaction tubes (diluted in 20µL water) | ||
| Line 497: | Line 507: | ||
**Primers were TolC_seq_F and TolC_seq_R. | **Primers were TolC_seq_F and TolC_seq_R. | ||
***Without insertion these two primers will create 449 bp sequence, and with insertion length will be 1650 bp | ***Without insertion these two primers will create 449 bp sequence, and with insertion length will be 1650 bp | ||
| - | [[File: | + | [[File: HARV2011.07.06.TolCinsertionconstruct+negativecontrol(labeled).png|thumb|none|TolC insert check 1 7/14/11]] |
**Some of the original culture after recombineering, 1ml solution spun down, and resuspended in 100μl of LB, and plated on new KAN plates. To check, one of the old KAN plates was plated the same way and put in at 2:30pm. (These plates will be checked tomorrow morning.) | **Some of the original culture after recombineering, 1ml solution spun down, and resuspended in 100μl of LB, and plated on new KAN plates. To check, one of the old KAN plates was plated the same way and put in at 2:30pm. (These plates will be checked tomorrow morning.) | ||
*PCR done of a few of the rpoZ MAGE with stop codon insertions. Oligo name: rpoZ_MAGE_KO (Noah's oligo) (Noah's plates, after two cycles of MAGE, on both ECNR2 and ECNR2ΔTolC). The side product in the gel could be due to a low annealing temperature of 53°C, so we should do a gradient PCR (e.g.55-66°C) to get the most product the next time. </div> | *PCR done of a few of the rpoZ MAGE with stop codon insertions. Oligo name: rpoZ_MAGE_KO (Noah's oligo) (Noah's plates, after two cycles of MAGE, on both ECNR2 and ECNR2ΔTolC). The side product in the gel could be due to a low annealing temperature of 53°C, so we should do a gradient PCR (e.g.55-66°C) to get the most product the next time. </div> | ||
 "
"