Team:Brown-Stanford/REGObricks/Biobrick
From 2011.igem.org
(→Biobrick2) |
(→Sequencing) |
||
| (67 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{:Team:Brown-Stanford/Templates/Main}} | {{:Team:Brown-Stanford/Templates/Main}} | ||
| + | <html> | ||
| + | <div id="subHeader"> | ||
| + | <ul id="subHeaderList"> | ||
| + | <li><a href="/Team:Brown-Stanford/REGObricks/Introduction">Introduction</a></li> | ||
| + | <li><a href="/Team:Brown-Stanford/REGObricks/ISRU">ISRU</a></li> | ||
| + | <li><a href="/Team:Brown-Stanford/REGObricks/Biocementation">Biocementation</a></li> | ||
| + | <li><a href="/Team:Brown-Stanford/REGObricks/Characterization"><em>S. pasteurii</em></a></li> | ||
| + | <li><a href="/Team:Brown-Stanford/REGObricks/Balloon">Balloon Flights</a></li> | ||
| + | <li><a href="/Team:Brown-Stanford/REGObricks/Transforming">Transformation</a></li> | ||
| + | <li id="active"><a href="#" id="current">Biobrick</html><sup>2</sup><html></a></li> | ||
| + | </ul> | ||
| + | </div> | ||
| + | </html> | ||
| + | {{:Team:Brown-Stanford/Templates/Content}} | ||
| + | == '''Biobrick<sup>2</sup> : Biobricking our Biobrick-er''' == | ||
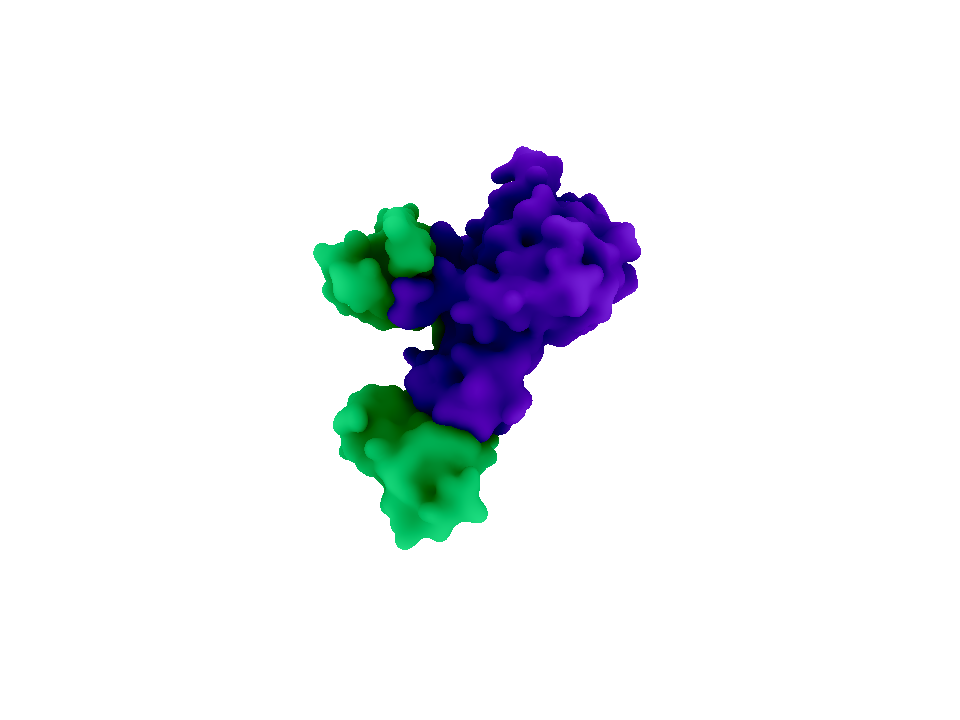
| - | + | [[File:Brown-Stanford Urease.png|thumb|450px|Urease (Image generated with Autodesk Maya)]] | |
| - | '''Research and acquisition''' | + | ==='''Research and acquisition'''=== |
| - | In June, we conducted background research on Sporosarcina pasteurii and | + | In June, we conducted background research on ''Sporosarcina pasteurii'' and its ureolytic ability. In these preliminary searches, we learned that the urease cassette of ''S. pasteurii'', responsible for the organism’s ureolysis and biocementation capabilities, had already been the subject of some cloning and transformations experiments involving ''E. coli''. {{:Team:Brown-Stanford/Templates/FootnoteNumber|1}}<sup>,</sup>{{:Team:Brown-Stanford/Templates/FootnoteNumber|2}}<sup>,</sup>{{:Team:Brown-Stanford/Templates/FootnoteNumber|3}} |
| - | + | ||
We contacted all of these investigators to learn the status of their current research in this area and to learn more about the urease cassette; unfortunately, many of these researchers were based in South Korea, and we never received a response to our inquires. Their research papers were similarly inaccesable on the internet. | We contacted all of these investigators to learn the status of their current research in this area and to learn more about the urease cassette; unfortunately, many of these researchers were based in South Korea, and we never received a response to our inquires. Their research papers were similarly inaccesable on the internet. | ||
| - | We lucked out when we contacted Dr. Sookie | + | We lucked out when we contacted [http://ssbang.sdsmt.edu/ Dr. Sookie Bang] of the South Dakota School of Mining and Technology, however; her lab had developed a number experiments studying microbially-induced calcite precipitation of both ''S. pasteurii'' and recombinant ''E. coli'', and still kept active strains of both. She and her graduate student, Terran Bergdale, generously sent us a plate of both ''S. pasteurii'' and HB101/pBU11 ''E.coli'', and provided us with wonderful advice and information throughout the entire summer. |
| - | The E. coli strain we were sent had been transformed with a plasmid containing the urease cassette of S. pasteurii and standard backbone pBR322. The sequence of pBR322 is freely available in many online databases, but the exact sequence of the urease cassette was unknown. As previously stated, we were unable to find or access the scientific literature that detailed the sequence or genes within the cassette. The only information we were able to obtain from | + | The ''E. coli'' strain we were sent had been transformed with a plasmid containing the urease cassette of ''S. pasteurii'' and standard backbone pBR322. The sequence of pBR322 is freely available in many online databases, but the exact sequence of the urease cassette was unknown. As previously stated, we were unable to find or access the scientific literature that detailed the sequence or genes within the cassette. The only information we were able to obtain from inquires and research was that the cassette itself was approximately 10.7 kb long, and was inserted into the HindIII restriction site of plasmid backbone pBR322. |
| - | + | ==='''Sequencing'''=== | |
| - | We | + | We put considerable energy into searching for the sequence in online databases; our search on NCBI yielded 3 distinct urease operons, none of which were the size of our cassette. Furthermore, we found few possible patterns of overlap among those NCBI results to predict a construct of 10.7 kb. |
| - | + | In the end we decided to pursue sequencing the construct sent to us. We were lucky enough to have met members of [http://physiology.med.cornell.edu/faculty/mason/lab/ the Mason Lab] of the Weill-Cornell Medical College earlier who generously offered to sequence the urease construct with their PacBio high-throughput sequencing platform. We graciously accepted and sent off copies of the original pBU11 plasmid for sequencing, but due to unforseen circumstances and poor luck with the platform and reagents we have not yet been able to obtain the sequence. We are now pursuing the sequence of the entire operon through more traditional primer walking, and we hope to have the entire data set available by MIT. | |
| - | + | ==='''Biobricking of Urease Cassette'''=== | |
| - | ''Primer Design'' - To clone the urease cassette out of pBR322 and modify it into a biobrick part, we designed sequence primers that would anneal to the template DNA but maintain an unbound DNA “tail” containing the appropriate restriction site sequences (E, X, S, P) for that end of the operon. EcoRI and XbaI were built into the forward primer, SpeI, PstI into the reverse | + | ''Primer Design'' - To clone the urease cassette out of pBR322 and modify it into a biobrick part, we designed sequence primers that would anneal to the template DNA but maintain an unbound DNA “tail” containing the appropriate restriction site sequences (E, X, S, P) for that end of the operon. EcoRI and XbaI were built into the forward primer, SpeI, PstI into the reverse. |
| - | Because we did not know the sequence of the urease cassette, | + | Because we did not know the sequence of the urease cassette, designing primers for the sequence would have been guesswork. Instead, we deigned the primers to anneal to the sequences of pBR322 that flanked both sides of the pBR322 HindIII cut site, into which the urease cassette had presumably been ligated. While this approach ultimately allowed us to biobrick a functional urease cassette form ''S. pasteurii'', the primers we designed were not optimal. First, the primers added approximately 30 bases each on either side of the cassette that were not originally part of the ''S. pasteurii'' operon, and these additions could have been avoided by previous knowledge of the sequence. Second, our primers included either side of the original pBR322 HindIII cutsite. In our characterization, we will determine whether this cut site was preserved in our biobrick and if it possible to cut our biobrick with HindIII. |
| - | ''HB101/pBU11'' - In order to clone out the urease cassette, we grew up, plated, and put in cryostock the HB101/pBU11 E. coli that we were sent by the Bang lab. Liquid cultures and plates were composed of LB | + | ''HB101/pBU11'' - In order to clone out the urease cassette, we grew up, plated, and put in cryostock the HB101/pBU11 ''E. coli'' that we were sent by the Bang lab. Liquid cultures and plates were composed of [[Team:Brown-Stanford/Lab/Protocols/LB]] and ampicillin, to which pBR322 confers resistance. |
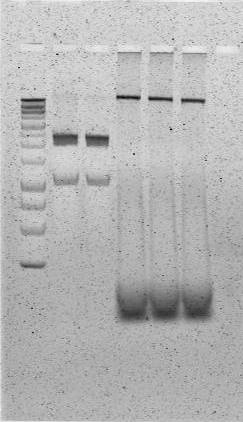
| - | + | [[File:Successful Phusion PCR Urease Cassette.jpg|300px|thumb|The gel image above represents a successful PCR amplification of the urease cassette insert of pUB11. The leftmost lane depicts a 1 kb ladder, and all lanes to the right of the ladder are PCR product. In each lane, the topmost band represents template plasmid (~15.1 kb), and the second band represents successfully amplified urease construct (~10.8 kb). A negative control had been performed on another PCR/gel verification protocol in parallel with the above workflow.]] | |
| - | [ | + | ''PCR'' - We used Phusion® High-Fidelity PCR Kit to develop a successful [https://2011.igem.org/Team:Brown-Stanford/Lab/Protocols/PhusionPCR PCR amplification protocol] for the urease cassette template. Because of the length of the desired sequence, we needed a polymerase capable of both long read length and high fidelity, which were both provided by the Phusion® kit. |
| - | The gel image | + | The gel image to the right represents a successful PCR amplification of the urease cassette insert of pUB11 using our edited Phusion PCR protocol. The leftmost lane depicts a 1 kb ladder, and all lanes to the right of the ladder are PCR product. In each lane, the topmost band represents template plasmid (~15.1 kb), and the second band represents successfully amplified urease construct (~10.8 kb). A negative control had been performed on another PCR/gel verification protocol in parallel with the above workflow. |
We then performed PCR purification with the Wizard® SV Gel and PCR Clean-Up System to prepare the samples for restriction digest. | We then performed PCR purification with the Wizard® SV Gel and PCR Clean-Up System to prepare the samples for restriction digest. | ||
| - | ''Restriction digest'' - Because we had no information concerning the urease cassette, we had no idea if any of the four standard biobrick restriction sites were present in its sequence. Therefore, we attempted restriction | + | ''Restriction digest'' - Because we had no information concerning the urease cassette, we had no idea if any of the four standard biobrick restriction sites were present in its sequence. Therefore, we attempted our [https://2011.igem.org/Team:Brown-Stanford/Lab/Protocol/BiobrickRestrictionDigest restriction digest protocol] in a random fashion, beginning with XbaI and PstI cuts: |
The gel above represents XbaI, PstI digests of plasmid pSCB1C3 (lane 3) and of our urease construct/PCR product (lane 4). Lanes 1 and 5 display 1 kb ladders; the last two lanes are spurious data. | The gel above represents XbaI, PstI digests of plasmid pSCB1C3 (lane 3) and of our urease construct/PCR product (lane 4). Lanes 1 and 5 display 1 kb ladders; the last two lanes are spurious data. | ||
| - | + | ||
XbaI, PstI restriction digests fragmented the urease cassette into four distinct bands at ~2.25, ~2.75, ~3, and ~4kb. These data suggest that at least two XbaI or PstI cut sites are present in the urease cassette itself, though it is difficult to locate them exactly as the digested results do not total the expected 10.8 kb. Later it was determined that the Pstl was responsible for the fragmentation displayed in the above image. | XbaI, PstI restriction digests fragmented the urease cassette into four distinct bands at ~2.25, ~2.75, ~3, and ~4kb. These data suggest that at least two XbaI or PstI cut sites are present in the urease cassette itself, though it is difficult to locate them exactly as the digested results do not total the expected 10.8 kb. Later it was determined that the Pstl was responsible for the fragmentation displayed in the above image. | ||
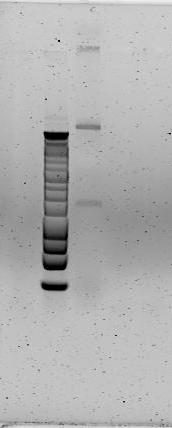
We conducted the same restriction digest protocol with XbaI, SpeI enzymes. The resulting product was much more encouraging as a single band still remained, at the same relative length as our urease construct. To test our restriction protocol, we again ran the same digest of pSB1C3 in parallel. | We conducted the same restriction digest protocol with XbaI, SpeI enzymes. The resulting product was much more encouraging as a single band still remained, at the same relative length as our urease construct. To test our restriction protocol, we again ran the same digest of pSB1C3 in parallel. | ||
| - | The above gel depicts 1 kb ladder (lane 1), XbaI, SpeI digests of pSB1C3 + J04450 (lanes 2, 3) and of the urease construct/PCR product (lanes 4, 5, 6). | + | [[File:X,S, restriction product (construct and plasmid).jpg|left|300px|thumb|The above gel depicts 1 kb ladder (lane 1), XbaI, SpeI digests of pSB1C3 + J04450 (lanes 2, 3) and of the urease construct/PCR product (lanes 4, 5, 6).]] [[File:Successful ligation product.jpg|300px|thumb|The above gel depicts a 1 kb ladder (lane 1) and the successful ligation of the urease cassette to pSB1C3.]] |
Because the urease construct does not seem to have diminished in size, and because there seems to only a single, strong band of a significant length, we assumed that both XbaI and SpeI were cutting only at their prescribed cut sites near the ends of the construct. It is possible that the restriction enzymes may cut inside the construct’s biobrick ends, theirin excising one half of the biobrick cut sites. Once sequencing is accomplished we will be able to determine if our digests was successful at preserving the biobrick ends of the construct. | Because the urease construct does not seem to have diminished in size, and because there seems to only a single, strong band of a significant length, we assumed that both XbaI and SpeI were cutting only at their prescribed cut sites near the ends of the construct. It is possible that the restriction enzymes may cut inside the construct’s biobrick ends, theirin excising one half of the biobrick cut sites. Once sequencing is accomplished we will be able to determine if our digests was successful at preserving the biobrick ends of the construct. | ||
| - | |||
''Gel Extraction'' - The XbaI, SpeI digests of both pSB1C3 and our urease construct were run through agarose gel through gel electrophoresis. Gel extraction was performed with the Wizard® SV Gel and PCR Clean-Up kit and protocol | ''Gel Extraction'' - The XbaI, SpeI digests of both pSB1C3 and our urease construct were run through agarose gel through gel electrophoresis. Gel extraction was performed with the Wizard® SV Gel and PCR Clean-Up kit and protocol | ||
| - | ''Ligation'' - We ligated our urease contruct with pSB1C3 plasmid backbone using T4 DNA | + | ''Ligation'' - We ligated our urease contruct with pSB1C3 plasmid backbone using [https://2011.igem.org/Team:Brown-Stanford/Lab/Protocol/T4Ligation T4 DNA ligation protocol]. |
| - | ''Transformation'' - Transformation of E. coli with our urease construct/pSCB3 (part BBa_K656013) was conducted using NEB 5-alpha Competent E. coli (High Efficiency). Transformant cells were plated on LB-chlor plates and allowed to grow overnight. Colonies were sampled at random, and tested for ureolytic activity. | + | ''Transformation'' - Transformation of ''E. coli'' with our urease construct/pSCB3 (part BBa_K656013) was conducted using NEB's [https://2011.igem.org/Team:Brown-Stanford/Lab/Protocols/NEB_5-alpha NEB 5-alpha Competent ''E. coli'' (High Efficiency) protocol]. Transformant cells were plated on LB-chlor plates and allowed to grow overnight. Colonies were sampled at random, and tested for ureolytic activity. |
| - | + | ||
| - | + | ||
'''Characterization''' | '''Characterization''' | ||
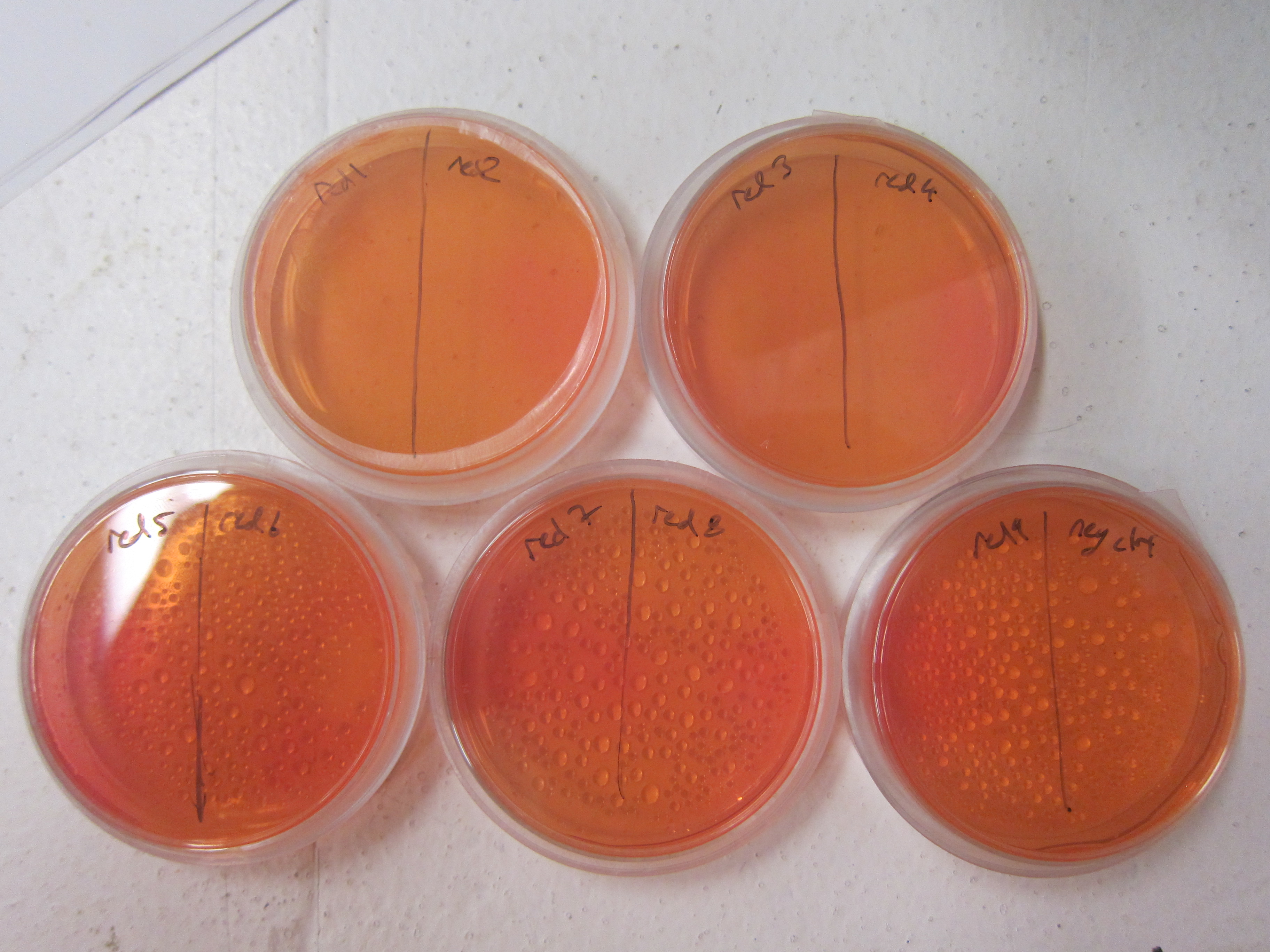
| - | As a qualitative assay of urease activity, we selected transformant colonies at random from the LB- | + | As a qualitative assay of urease activity, we selected transformant colonies at random from the LB-chlor growth plates and grew them up in liquid LB-chlor culture. These monoclonal cultures were then plated and allowed to grow on [https://2011.igem.org/Team:Brown-Stanford/Lab/Protocols/UreaseTestPlates urease test plates]; if urease is present and active in the cultures, the urea is cleaved in the surrounding agar, causing the pH of the surrounding agar to increase. Phenol-red then indicates the subsequent increase in pH from ureolysis. |
| - | All but one of the colonies we selected off the LB-Chlor plate exhibited ureolytic activity, indicating that our workflow had resulted in the construction of a functional S. pasteurii urease biobrick. From the urease test plates | + | All but one of the colonies we selected off the LB-Chlor plate exhibited ureolytic activity, '''indicating that our workflow had resulted in the construction of a functional ''S. pasteurii'' urease biobrick.''' From the urease test plates we were able to determine that our part did in fact, function, albeit qualititatively. |
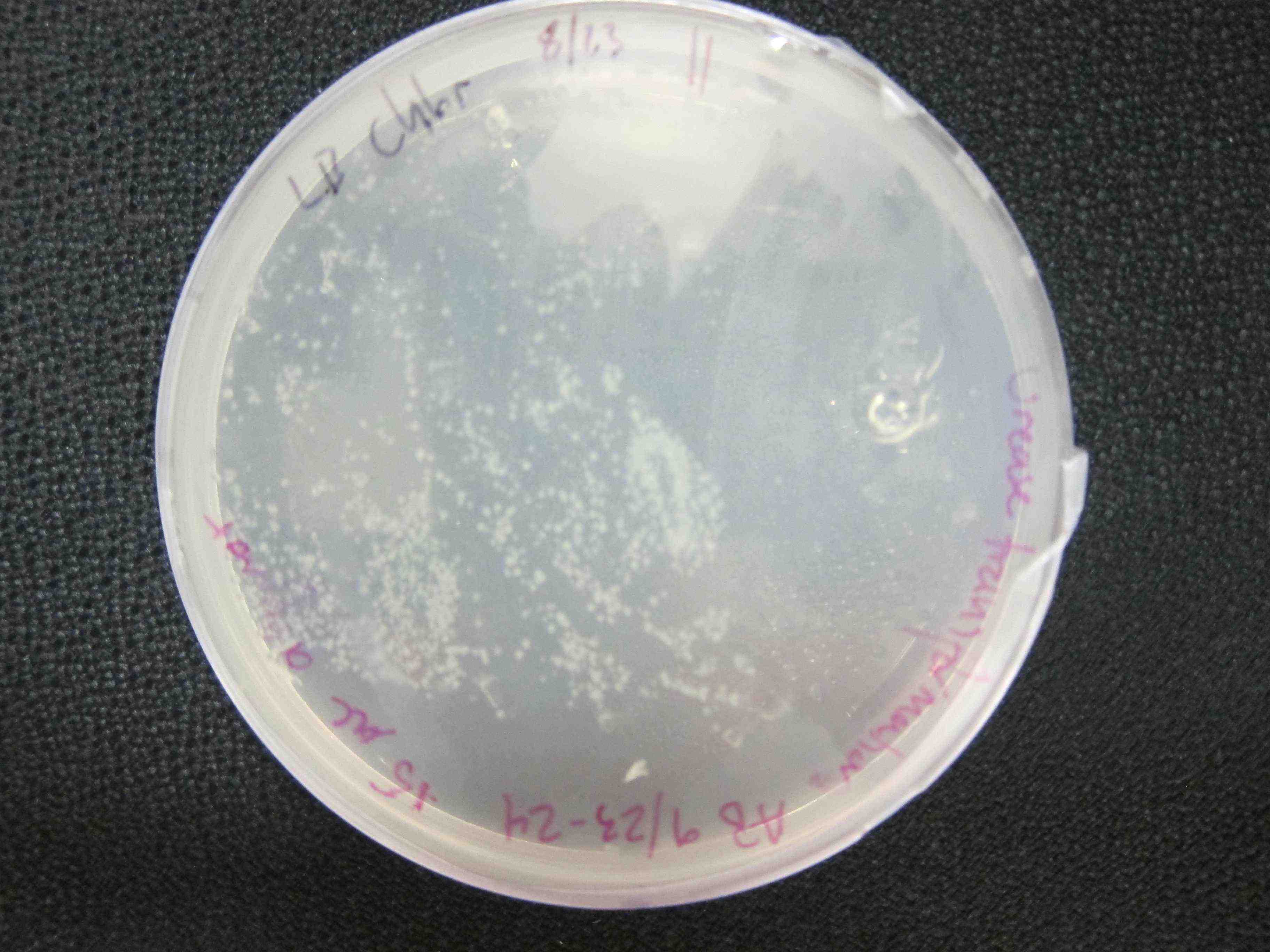
| - | To more accurately and quantitatively measure the activity of the urease biobrick, we | + | [[File:Successful Transformation urease biobrick.jpg|left|300px|thumb|Successful urease biobrick transformants, plated on LB-chlor agar plates.]] [[File:Transformant urease plates.JPG|right|300px|thumb|Successful urease biobrick transformants, plated on urease test plates.]] |
| + | |||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | To more accurately and quantitatively measure the activity of the urease biobrick, we hope to perform a [https://2011.igem.org/Team:Brown-Stanford/Lab/Protocols/Conductivity conductivity assay protocol] with our transformants. At this time, however, the conductivity meter is not available in our building has gone missing; we are doing all that we can to retrieve it, and once in hand we will perform the assay and upload the results to [[http://partsregistry.org/Part:BBa_K656013|part BBa_K656013's page] in the registry. | ||
| + | |||
| + | Another component of our characterization of part BBa_K656013 was a determination of the construct's compatibility with the biobrick assembly method. Because we lack the sequence data for this part, we performed the basic biobrick restriction digests (EcoRI, XbaI ,SpeI , and PstI) on our urease construct to determine if there were any cut sites within the operon itself. | ||
| + | Our construct did indeed contain multiple biobrick restriction sites, specifically EcoRI and PstI, both of which cut our operon into multiple pieces. Again, because of our lack of a sequence for this part, it is impossible to say for sure where in the operon these cut sites exist; fortunately, neither XbaI nor SpeI enzymes cut our operon into distinct pieces, allowing us to incorporate the construct into biobrick submission plasmid pSB1C3. Therefore, the part we created is compatible with standard biobrick assembly, but should not be used with the outer restriction enzymes EcoRI and PstI. | ||
| + | For the most up-to-date characterization and data concerning part BBa_K656013, please visit the part's page [http://partsregistry.org/Part:BBa_K656013 here] | ||
| + | |||
| + | |||
| + | '''Judging''' | ||
| + | *Successfully cloned and biobricked complete ''Sporosarcina pasteurii'' urease operon. This construct was successfully transformed into 5-alpha competent ''E. coli'' with plasmid backbone/vector pSB1C3. The biobricked urease operon has been added to the Registry of Standard Biological Parts as [http://partsregistry.org/Part:BBa_K656013 Part:BBa_K656013]. | ||
| + | *Qualitatively characterized and demonstrated the ureolytic functionality of part BBa_K656013 in 5-alpha ''E. coli''. | ||
| + | *Are working to quantitatively record the ureolytic activity of ''E. coli'' transformants expressing part BBa-K656013 through our conductivity protocol, and will post this data to the part's page in the registry | ||
| + | *Characterized standard biobrick restriction sites within part BBa-K656013. The part contains EcoRI and PstI cut sites besides those intended for standard biobrick assembly. Therefore, any construct or assembly should utilize only XbaI and SpeI restriction enzymes when working with part BBa_K656013. | ||
| + | |||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | |||
| + | ===References=== | ||
| + | |||
| + | {{:Team:Brown-Stanford/Templates/Footnote|1| Kim, S.D. and Hausinger, R.P. (1994) Genetic organization of the recombinant ''Bacillus pasteurii'' urease genes expressed in ''Escherichia coli''. Journal of Microbiology and Biotechnology 4: 108-112.}} | ||
| + | |||
| + | {{:Team:Brown-Stanford/Templates/Footnote|2| You, J.H., Kim, J.G., Song, B.H., Lee, M.H. and Kim, S.D. (1995) Genetic organization and nucleotide sequence of the ure gene cluster in ''Bacillus pasteurii.'' Molecular Cells 5: 359-369.}} | ||
| + | {{:Team:Brown-Stanford/Templates/Footnote|3| Bang, S.S. and Ramakrishnan, V. (S.S. Bang, J.K. Galinat and V. Ramakrishnan, Calcite precipitation induced by polyurethane-immobilized ''Bacillus pasteurii''. Enzyme and Microbial Technology, 28 4 (2001), pp. 404–409.}} | ||
{{:Team:Brown-Stanford/Templates/Foot}} | {{:Team:Brown-Stanford/Templates/Foot}} | ||
Latest revision as of 01:31, 29 October 2011
Biobrick2 : Biobricking our Biobrick-er
Research and acquisition
In June, we conducted background research on Sporosarcina pasteurii and its ureolytic ability. In these preliminary searches, we learned that the urease cassette of S. pasteurii, responsible for the organism’s ureolysis and biocementation capabilities, had already been the subject of some cloning and transformations experiments involving E. coli. 1,2,3
We contacted all of these investigators to learn the status of their current research in this area and to learn more about the urease cassette; unfortunately, many of these researchers were based in South Korea, and we never received a response to our inquires. Their research papers were similarly inaccesable on the internet. We lucked out when we contacted [http://ssbang.sdsmt.edu/ Dr. Sookie Bang] of the South Dakota School of Mining and Technology, however; her lab had developed a number experiments studying microbially-induced calcite precipitation of both S. pasteurii and recombinant E. coli, and still kept active strains of both. She and her graduate student, Terran Bergdale, generously sent us a plate of both S. pasteurii and HB101/pBU11 E.coli, and provided us with wonderful advice and information throughout the entire summer. The E. coli strain we were sent had been transformed with a plasmid containing the urease cassette of S. pasteurii and standard backbone pBR322. The sequence of pBR322 is freely available in many online databases, but the exact sequence of the urease cassette was unknown. As previously stated, we were unable to find or access the scientific literature that detailed the sequence or genes within the cassette. The only information we were able to obtain from inquires and research was that the cassette itself was approximately 10.7 kb long, and was inserted into the HindIII restriction site of plasmid backbone pBR322.
Sequencing
We put considerable energy into searching for the sequence in online databases; our search on NCBI yielded 3 distinct urease operons, none of which were the size of our cassette. Furthermore, we found few possible patterns of overlap among those NCBI results to predict a construct of 10.7 kb. In the end we decided to pursue sequencing the construct sent to us. We were lucky enough to have met members of [http://physiology.med.cornell.edu/faculty/mason/lab/ the Mason Lab] of the Weill-Cornell Medical College earlier who generously offered to sequence the urease construct with their PacBio high-throughput sequencing platform. We graciously accepted and sent off copies of the original pBU11 plasmid for sequencing, but due to unforseen circumstances and poor luck with the platform and reagents we have not yet been able to obtain the sequence. We are now pursuing the sequence of the entire operon through more traditional primer walking, and we hope to have the entire data set available by MIT.
Biobricking of Urease Cassette
Primer Design - To clone the urease cassette out of pBR322 and modify it into a biobrick part, we designed sequence primers that would anneal to the template DNA but maintain an unbound DNA “tail” containing the appropriate restriction site sequences (E, X, S, P) for that end of the operon. EcoRI and XbaI were built into the forward primer, SpeI, PstI into the reverse. Because we did not know the sequence of the urease cassette, designing primers for the sequence would have been guesswork. Instead, we deigned the primers to anneal to the sequences of pBR322 that flanked both sides of the pBR322 HindIII cut site, into which the urease cassette had presumably been ligated. While this approach ultimately allowed us to biobrick a functional urease cassette form S. pasteurii, the primers we designed were not optimal. First, the primers added approximately 30 bases each on either side of the cassette that were not originally part of the S. pasteurii operon, and these additions could have been avoided by previous knowledge of the sequence. Second, our primers included either side of the original pBR322 HindIII cutsite. In our characterization, we will determine whether this cut site was preserved in our biobrick and if it possible to cut our biobrick with HindIII.
HB101/pBU11 - In order to clone out the urease cassette, we grew up, plated, and put in cryostock the HB101/pBU11 E. coli that we were sent by the Bang lab. Liquid cultures and plates were composed of Team:Brown-Stanford/Lab/Protocols/LB and ampicillin, to which pBR322 confers resistance.
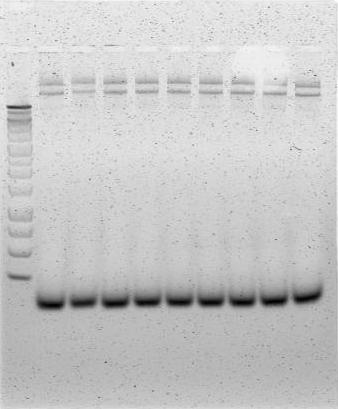
PCR - We used Phusion® High-Fidelity PCR Kit to develop a successful PCR amplification protocol for the urease cassette template. Because of the length of the desired sequence, we needed a polymerase capable of both long read length and high fidelity, which were both provided by the Phusion® kit.
The gel image to the right represents a successful PCR amplification of the urease cassette insert of pUB11 using our edited Phusion PCR protocol. The leftmost lane depicts a 1 kb ladder, and all lanes to the right of the ladder are PCR product. In each lane, the topmost band represents template plasmid (~15.1 kb), and the second band represents successfully amplified urease construct (~10.8 kb). A negative control had been performed on another PCR/gel verification protocol in parallel with the above workflow.
We then performed PCR purification with the Wizard® SV Gel and PCR Clean-Up System to prepare the samples for restriction digest.
Restriction digest - Because we had no information concerning the urease cassette, we had no idea if any of the four standard biobrick restriction sites were present in its sequence. Therefore, we attempted our restriction digest protocol in a random fashion, beginning with XbaI and PstI cuts: The gel above represents XbaI, PstI digests of plasmid pSCB1C3 (lane 3) and of our urease construct/PCR product (lane 4). Lanes 1 and 5 display 1 kb ladders; the last two lanes are spurious data.
XbaI, PstI restriction digests fragmented the urease cassette into four distinct bands at ~2.25, ~2.75, ~3, and ~4kb. These data suggest that at least two XbaI or PstI cut sites are present in the urease cassette itself, though it is difficult to locate them exactly as the digested results do not total the expected 10.8 kb. Later it was determined that the Pstl was responsible for the fragmentation displayed in the above image. We conducted the same restriction digest protocol with XbaI, SpeI enzymes. The resulting product was much more encouraging as a single band still remained, at the same relative length as our urease construct. To test our restriction protocol, we again ran the same digest of pSB1C3 in parallel.
Because the urease construct does not seem to have diminished in size, and because there seems to only a single, strong band of a significant length, we assumed that both XbaI and SpeI were cutting only at their prescribed cut sites near the ends of the construct. It is possible that the restriction enzymes may cut inside the construct’s biobrick ends, theirin excising one half of the biobrick cut sites. Once sequencing is accomplished we will be able to determine if our digests was successful at preserving the biobrick ends of the construct.
Gel Extraction - The XbaI, SpeI digests of both pSB1C3 and our urease construct were run through agarose gel through gel electrophoresis. Gel extraction was performed with the Wizard® SV Gel and PCR Clean-Up kit and protocol
Ligation - We ligated our urease contruct with pSB1C3 plasmid backbone using T4 DNA ligation protocol.
Transformation - Transformation of E. coli with our urease construct/pSCB3 (part BBa_K656013) was conducted using NEB's NEB 5-alpha Competent E. coli (High Efficiency) protocol. Transformant cells were plated on LB-chlor plates and allowed to grow overnight. Colonies were sampled at random, and tested for ureolytic activity.
Characterization
As a qualitative assay of urease activity, we selected transformant colonies at random from the LB-chlor growth plates and grew them up in liquid LB-chlor culture. These monoclonal cultures were then plated and allowed to grow on urease test plates; if urease is present and active in the cultures, the urea is cleaved in the surrounding agar, causing the pH of the surrounding agar to increase. Phenol-red then indicates the subsequent increase in pH from ureolysis. All but one of the colonies we selected off the LB-Chlor plate exhibited ureolytic activity, indicating that our workflow had resulted in the construction of a functional S. pasteurii urease biobrick. From the urease test plates we were able to determine that our part did in fact, function, albeit qualititatively.
To more accurately and quantitatively measure the activity of the urease biobrick, we hope to perform a conductivity assay protocol with our transformants. At this time, however, the conductivity meter is not available in our building has gone missing; we are doing all that we can to retrieve it, and once in hand we will perform the assay and upload the results to [[http://partsregistry.org/Part:BBa_K656013|part BBa_K656013's page] in the registry.
Another component of our characterization of part BBa_K656013 was a determination of the construct's compatibility with the biobrick assembly method. Because we lack the sequence data for this part, we performed the basic biobrick restriction digests (EcoRI, XbaI ,SpeI , and PstI) on our urease construct to determine if there were any cut sites within the operon itself. Our construct did indeed contain multiple biobrick restriction sites, specifically EcoRI and PstI, both of which cut our operon into multiple pieces. Again, because of our lack of a sequence for this part, it is impossible to say for sure where in the operon these cut sites exist; fortunately, neither XbaI nor SpeI enzymes cut our operon into distinct pieces, allowing us to incorporate the construct into biobrick submission plasmid pSB1C3. Therefore, the part we created is compatible with standard biobrick assembly, but should not be used with the outer restriction enzymes EcoRI and PstI. For the most up-to-date characterization and data concerning part BBa_K656013, please visit the part's page [http://partsregistry.org/Part:BBa_K656013 here]
Judging
- Successfully cloned and biobricked complete Sporosarcina pasteurii urease operon. This construct was successfully transformed into 5-alpha competent E. coli with plasmid backbone/vector pSB1C3. The biobricked urease operon has been added to the Registry of Standard Biological Parts as [http://partsregistry.org/Part:BBa_K656013 Part:BBa_K656013].
- Qualitatively characterized and demonstrated the ureolytic functionality of part BBa_K656013 in 5-alpha E. coli.
- Are working to quantitatively record the ureolytic activity of E. coli transformants expressing part BBa-K656013 through our conductivity protocol, and will post this data to the part's page in the registry
- Characterized standard biobrick restriction sites within part BBa-K656013. The part contains EcoRI and PstI cut sites besides those intended for standard biobrick assembly. Therefore, any construct or assembly should utilize only XbaI and SpeI restriction enzymes when working with part BBa_K656013.
References
1 Kim, S.D. and Hausinger, R.P. (1994) Genetic organization of the recombinant Bacillus pasteurii urease genes expressed in Escherichia coli. Journal of Microbiology and Biotechnology 4: 108-112.
2 You, J.H., Kim, J.G., Song, B.H., Lee, M.H. and Kim, S.D. (1995) Genetic organization and nucleotide sequence of the ure gene cluster in Bacillus pasteurii. Molecular Cells 5: 359-369.
3 Bang, S.S. and Ramakrishnan, V. (S.S. Bang, J.K. Galinat and V. Ramakrishnan, Calcite precipitation induced by polyurethane-immobilized Bacillus pasteurii. Enzyme and Microbial Technology, 28 4 (2001), pp. 404–409.
 "
"