Team:Wageningen UR/Project/DevicesSetup
From 2011.igem.org
RubenvanHeck (Talk | contribs) (→Custom fluidic device designed by Team Wageningen UR to measure oscillations) |
RubenvanHeck (Talk | contribs) m (→Custom fluidic device designed by Team Wageningen UR to measure oscillations) |
||
| Line 27: | Line 27: | ||
[[File:Hasty_device_WUR.png|200px|center]] | [[File:Hasty_device_WUR.png|200px|center]] | ||
[ref][[File:Legend_device_WUR.png|140px|center]] | [ref][[File:Legend_device_WUR.png|140px|center]] | ||
| - | '''Fig. | + | |
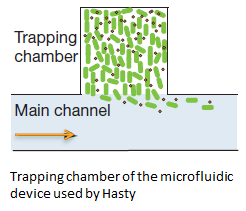
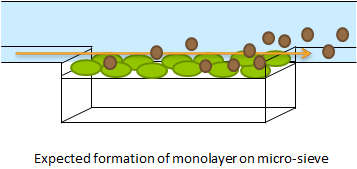
| - | The micro-sieve seemed promising for creating a monolayer of cells. The cells could be drawn toward the sieve by applying an under pressure (for example with a syringe). Since the Top10 ''E.coli'' strain used for our transformations does not form biofilms, additional cells would be flushed away once all the pores of the micro-sieve are blocked. However, the flow over this monolayer is in a different dimensional plane (compare figure 1 & 2). The direction of the flow over the micro-sieve can be seen in figure 2. | + | '''Fig.1''' The micro-sieve seemed promising for creating a monolayer of cells. The cells could be drawn toward the sieve by applying an under pressure (for example with a syringe). Since the Top10 ''E.coli'' strain used for our transformations does not form biofilms, additional cells would be flushed away once all the pores of the micro-sieve are blocked. However, the flow over this monolayer is in a different dimensional plane (compare figure 1 & 2). The direction of the flow over the micro-sieve can be seen in figure 2. |
[[File:Micro-sieve_device_WUR.png|270px|center]] | [[File:Micro-sieve_device_WUR.png|270px|center]] | ||
| - | + | '''Fig.2:'''This setup imposes the problem that the diffusion of AHL is much slower than the flow rate over the sieve. Therefore, the AHL produced will always be flushed away before a uniform concentration can be established over the whole cell culture, thus preventing any synchronized behavior to arise. The use of a microsieve in the course of our iGEM project was therefore discarded. | |
| - | This setup imposes the problem that the diffusion of AHL is much slower than the flow rate over the sieve. Therefore, the AHL produced will always be flushed away before a uniform concentration can be established over the whole cell culture, thus preventing any synchronized behavior to arise. The use of a microsieve in the course of our iGEM project was therefore discarded. | + | |
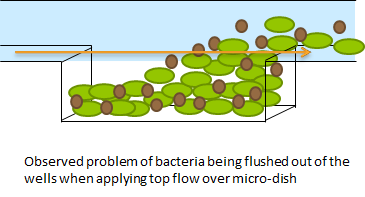
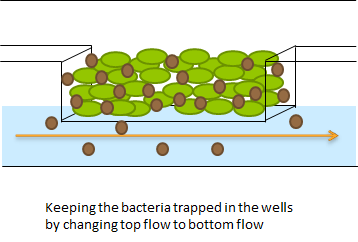
The problem described above does not arise when using the microdish. In the 40 micron deep wells the cells can be trapped and AHL will have a better chance to establish a uniform concentration throughout the well. This will create a higher chance of synchronized oscillatory behavior of the cells growing in a well. Special care has to be taken with the velocities of the fluid flowing over the wells: If the flow rate is too high, the cells will be spilled out of the wells. This behavior was observed under the microscope. | The problem described above does not arise when using the microdish. In the 40 micron deep wells the cells can be trapped and AHL will have a better chance to establish a uniform concentration throughout the well. This will create a higher chance of synchronized oscillatory behavior of the cells growing in a well. Special care has to be taken with the velocities of the fluid flowing over the wells: If the flow rate is too high, the cells will be spilled out of the wells. This behavior was observed under the microscope. | ||
| Line 39: | Line 38: | ||
[[File:Micro-dish1_device_WUR.png|290px]] [[File:Flushingoutofwells_WUR.gif|300px|right]] | [[File:Micro-dish1_device_WUR.png|290px]] [[File:Flushingoutofwells_WUR.gif|300px|right]] | ||
| - | + | '''Fig.3''' ‘’’Fig.4’’’ | |
| Line 50: | Line 49: | ||
[[File:Micro-dish2_device_WUR.png|200px]] [[File:Bottom_feed_WUR.png|500px|right]] | [[File:Micro-dish2_device_WUR.png|200px]] [[File:Bottom_feed_WUR.png|500px|right]] | ||
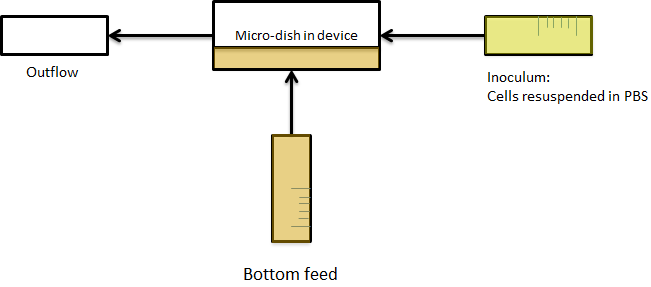
| - | + | '''Fig.5''' ''Applying bottom feeding to keep the cells in the wells alive'' '''Fig.6''' | |
| Line 90: | Line 89: | ||
[[File:Measuring_GFP_WUR.jpg|500px|center]] | [[File:Measuring_GFP_WUR.jpg|500px|center]] | ||
| - | + | '''Fig.9''' | |
For the experiments, an overnight culture of the cells containing our construct was spun down and resuspended in PBS. The resuspended culture was inoculated in the device and left in the chamber to settle down for a while. Since the bacteria were bottom fed with LB as seen in the setup section in [[Team:Wageningen_UR/Project/DevicesSetup#Controlling_cell_growth| figure 6]], only the bacteria which settled down in the wells survived, while the bacteria in PBS starved to death. This is shown in the short video below. The pictures were taken every ten minutes. | For the experiments, an overnight culture of the cells containing our construct was spun down and resuspended in PBS. The resuspended culture was inoculated in the device and left in the chamber to settle down for a while. Since the bacteria were bottom fed with LB as seen in the setup section in [[Team:Wageningen_UR/Project/DevicesSetup#Controlling_cell_growth| figure 6]], only the bacteria which settled down in the wells survived, while the bacteria in PBS starved to death. This is shown in the short video below. The pictures were taken every ten minutes. | ||
| Line 101: | Line 100: | ||
[[File:ptetGFP_PBS.jpg|350px]] [[File:ptetGFP_PBSremoved.jpg|350px|right]] | [[File:ptetGFP_PBS.jpg|350px]] [[File:ptetGFP_PBSremoved.jpg|350px|right]] | ||
| - | + | '''Fig.10''' ‘’Before addition of ‘’After removal of PBS’’ '''Fig.11''' | |
Revision as of 19:34, 20 September 2011
 "
"