Team:Wageningen UR/Project/DevicesMeasuringOscillations
From 2011.igem.org
(→Customary fluidic device designed by Team Wageningen UR to measure oscillations) |
(→Customary fluidic device designed by Team Wageningen UR to measure oscillations) |
||
| (18 intermediate revisions not shown) | |||
| Line 19: | Line 19: | ||
{{:Team:Wageningen_UR/Templates/Header}} | {{:Team:Wageningen_UR/Templates/Header}} | ||
{{:Team:Wageningen_UR/Templates/NavigationTop1}} | {{:Team:Wageningen_UR/Templates/NavigationTop1}} | ||
| - | == | + | == Custom fluidic device designed by Team Wageningen UR to measure oscillations == |
{{:Team:Wageningen_UR/Templates/NavigationLeftDevice}} | {{:Team:Wageningen_UR/Templates/NavigationLeftDevice}} | ||
{{:Team:Wageningen_UR/Templates/Style | text= __NOTOC__ | {{:Team:Wageningen_UR/Templates/Style | text= __NOTOC__ | ||
| Line 26: | Line 26: | ||
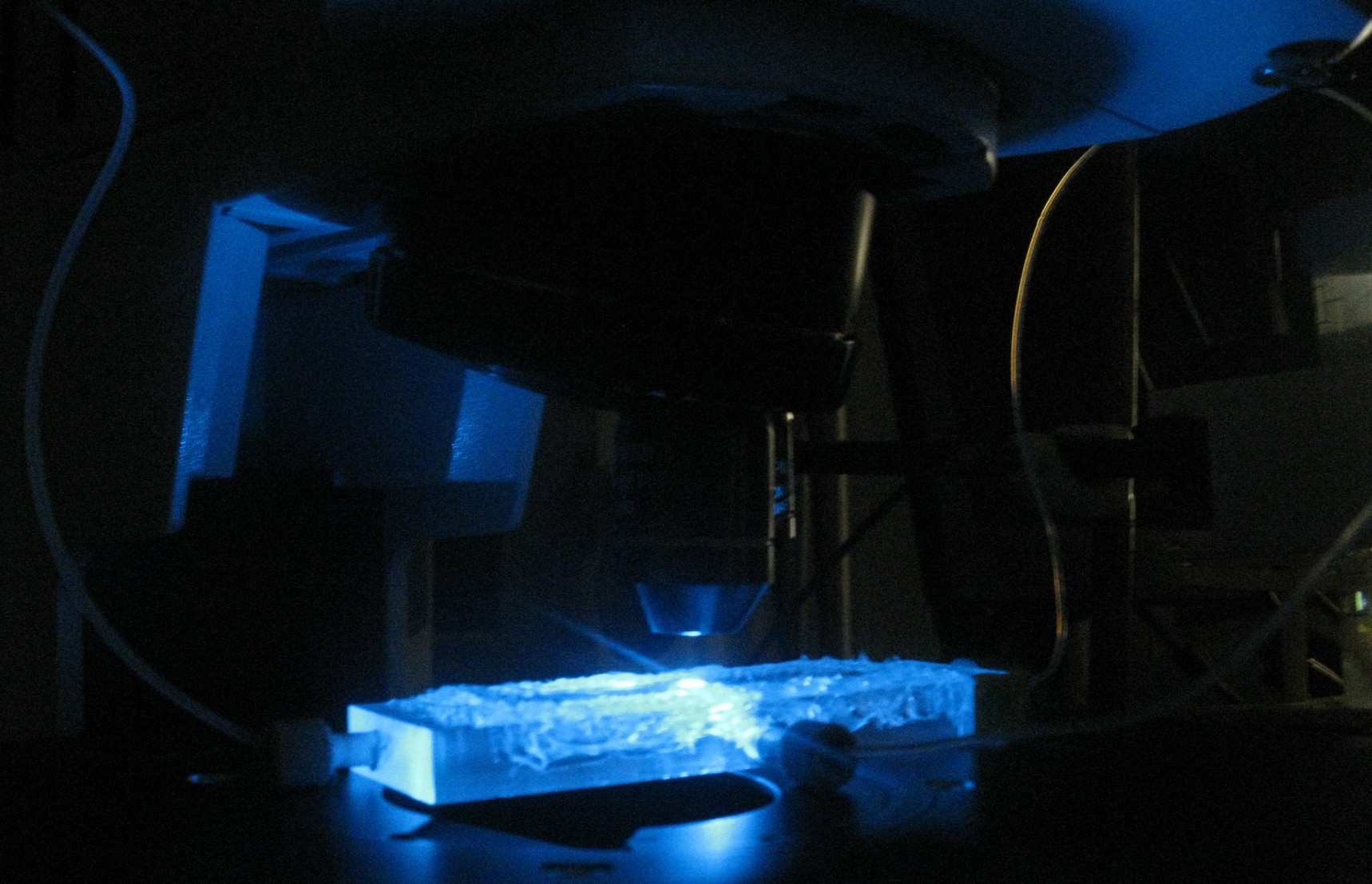
[[File:Measuring_GFP_WUR.jpg|500px|center]] | [[File:Measuring_GFP_WUR.jpg|500px|center]] | ||
| + | By chance an oscillatory behaviour of transfomed ''E.coli'' containing the [[Team:Wageningen_UR/Project/CompleteProject1Description#Streamlined_Design| streamlined construct]] was observed in one of the experiments performed with a plate reader. This suggested oscillations could occur even without applying any flow over the wells. Letting the [[Team:Wageningen_UR/Project/ModelingProj1#Writing_a_modeling_tool_in_matlab| modeling tool]] iterate over a range of cell desities while keeping the flow rate constant at 0 confirmed that [[Team:Wageningen_UR/Project/ModelingProj1#Conclusions_for_our_system| oscillations could occur]] at high cell densities. Therefore the measurements for oscillatory behaviour of the construct were taken without applying any flow. | ||
| - | |||
| + | For the experiments, an overnight culture of the cells containing the relevant construct was spun down and resuspended in PBS. The resuspended culture was inoculated in the device and left in the chamber to settle down for a while. Since the bacteria were bottom fed with LB as seen in the set up section in [[Team:Wageningen_UR/Project/DevicesSetup#Controlling_cell_growth| figure X+2]], only the bacteria which settled down in the wells survived, while the bacteria in PBS starved to death. This is shown in the short video below. The pictures were taken every ten minutes. | ||
| - | [[File: | + | [[File:PBS_cellgrowth_bottomfeed-1.tif|center]] |
| + | The video shows the micro-dish directly after inoculation, when the bacteria are still floating around everywhere in the chamber. After that the bacteria which are only in PBS start to die, whereas the bacteria in the wells survive. | ||
| - | + | After letting the bacteria grow in this manner for 2-3 hours, the PBS was removed with the same syringe that was used to inoculate. The chamber was then let to dry out over night. The two pictures seen below show the micro-dish with a ptet-GFP strain growing in the wells directly before and after removal of the PBS. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | [[File:ptetGFP_PBS.jpg|350px]] [[File:ptetGFP_PBSremoved.jpg|350px|right]] | |
| + | For the experiment seen above, the PBS was removed before all the cells died. The procedure varied depending on how well the bacteria grew in the wells. They were left to grow in the device for an additional night and the measurements were then taken in an 10 minute intervall during the next day. This was done for two reasons, for one the chamber had to be completely dried out before measurements could be taken, otherwise the remaining liquid would condense through the heat of the light and blurr the pictures. The second reason was that, as mentioned before, [[Team:Wageningen_UR/Project/ModelingProj1#Conclusions_for_our_system| modeling]] suggested that, when applying no flow, the oscillations would occur only when starting with a high cell density. | ||
Latest revision as of 16:09, 19 September 2011
 "
"