Team:XMU-China/Labjournal
From 2011.igem.org
Contents |
Wetlab journal
Week 1(3rd Apr.—9th Apr.)
Aim:
By replacing the promoter of the existing BioBrick BBa_F2621 with Placo-1, the expression of the downstream sequence can be controlled by adding IPTG.
Performance:
Constructing the new BioBrick BBa_K658000 (The figure below and sequence analysis can indicate it is correctly constructed.)
Testing the expression of BBa_K658000 by adding IPTG (We thought the part didn’t exist in the registry and we had constructed a new part. But, afterwards, we found it(BBa_F2622) did exist! )

Week 2(10th Apr.—16th Apr.)
Aim:
By combining the BioBrick BBa_R0011, BBa_F1610 and BBa_K65800, we can get a new BioBrick which is part of the bacteria population-control device we have planned to design.
Performance:
Constructing the new BioBrick IR(BBa_K658012)
Week 3(17th Apr.—23rd Apr.)
Aim:
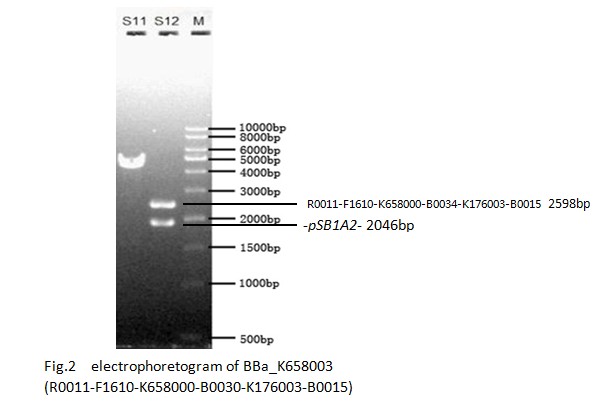
By adding the killer gene to the Biobrick IR, we can finally get the bacteria population-control device H(BBa_K658003).
Performance:
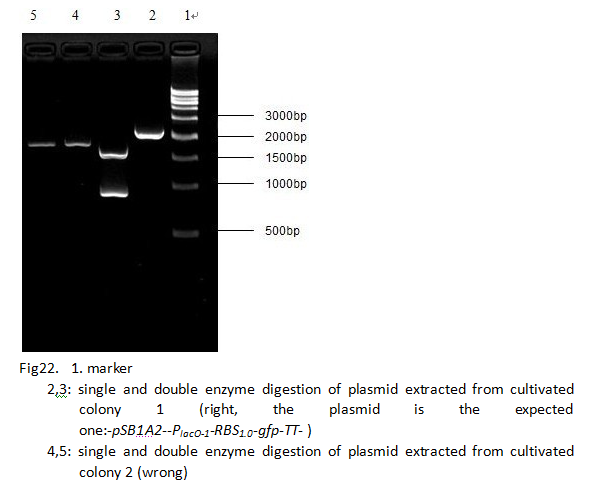
Constructing the BioBrick H. The figure shown below and sequence analysis can indicate that it is correctly constructed.

Week 4(24th Apr.—30th Apr.)
Aim:
We plan to construct a new part in order to test how the concentration of AHL can affect the expression of the downstream sequence under the control of lux pR. Performance:
Successfully construct the new part BBa_K658022 Testing on the performance of the part using different concentrations of AHL as inducer.The result is shown as follows:

Week 5-8(1st May—28th May)
Aim:
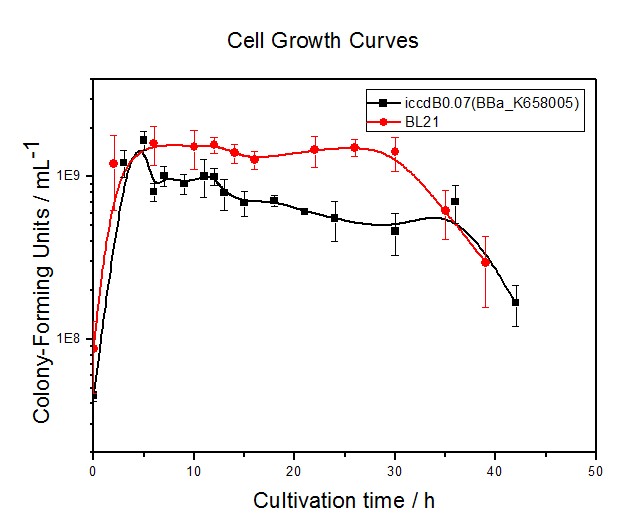
In order to investigate the performance of the device(iccdB) under different conditions, we plan to construct several devices with RBS of different efficiency to see how RBS can affect the functioning of the device.
Performance:
Week 5-7(1st May—21st May)
Constructing the population-control device with RBS0.07

S1: single endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxI-TT
S2: double endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxI-TT
S3: single endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxR-TT-lux pR
S4: double endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxR-TT-lux pR
S5: single endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxI-TT-PlacO-1-RBS1.0-luxR-TT-lux pR
S6: double endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxI-TT-PlacO-1-RBS1.0-luxR-TT-lux pR
S7: single endonuclease digestion of pSB1AK3-lacZɑ-ccdB-TT
S8: double endonuclease digestion of pSB1AK3-lacZɑ-ccdB-TT
S9: single endonuclease digestion of pSB1A2-RBS0.07-lacZɑ-ccdB-TT
S10: double endonuclease digestion of pSB1A2-RBS0.07-lacZɑ-ccdB-TT
S11: single endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxI-TT-PlacO-1-RBS1.0-luxR-TT-luxpR-RBS0.07-lacZɑ-ccdB-TT
S12: double endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxI-TT-PlacO-1-RBS1.0-luxR-TT-luxpR-RBS0.07-lacZɑ-ccdB-TT
Constructing the population-control device with RBS1.0

S1: single endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxI-TT;
S2: double endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxI-TT ;
S3: single endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxR-TT-lux pR
S4: double endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxR-TT-lux pR
S5: single endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxI-TT-PlacO-1-RBS1.0-luxR-TT-lux pR
S6: double endonuclease digestion Of pSB1A2-PlacO-1-RBS1.0-luxI-TT-PlacO-1-RBS1.0-luxR-TT-lux pR
S7: single endonuclease digestion of pSB1A2-RBS0.6-lacZɑ-ccdB
S8: double endonuclease digestion of pSB1A2-RBS0.6-lacZɑ-ccdB
S9: single endonuclease digestion Of pSB1A2-PlacO-1-RBS1.0-luxI-TT-PlacO-1-RBS1.0-luxR-TT-luxpR-RBS0.6-lacZɑ-ccdB
S10: double endonuclease digestion Of pSB1A2-PlacO-1-RBS1.0-luxI-TT-PlacO-1-RBS1.0-luxR-TT-luxpR-RBS0.6-lacZɑ-ccdB
S11: single endonuclease digestion Of pSB1A2-PlacO-1-RBS1.0-luxI-TT-PlacO-1-RBS1.0-luxR-TT-luxpR-RBS0.6-lacZɑ-ccdB
S12: double endonuclease digestion Of pSB1A2-PlacO-1-RBS1.0-luxI-TT-PlacO-1-RBS1.0-luxR-TT-luxpR-RBS0.6-lacZɑ-ccdB
Constructing the population-control device with RBS0.3

S1: single endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxI-TT
S2: double endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxI-TT
S3: single endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxR-TT-lux pR
S4: double endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxR-TT-lux pR
S5: single endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxI-TT-PlacO-1-RBS1.0-luxR-TT-lux pR
S6: double endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxI-TT-PlacO-1-RBS1.0-luxR-TT-lux pR
S7: single endonuclease digestion of pSB1A2-RBS0.3-lacZɑ-ccdB
S8: double endonuclease digestion of pSB1A2-RBS0.3-lacZɑ-ccdB
S9: single endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxI-TT-PlacO-1-RBS1.0-luxR-TT-luxpR-RBS0.3-lacZɑ-ccdB
S10: double endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxI-TT-PlacO-1-RBS1.0-luxR-TT-luxpR-RBS0.3-lacZɑ-ccdB
S11: single endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxI-TT-PlacO-1-RBS1.0-luxR-TT-luxpR-RBS0.3-lacZɑ-ccdB
S12: double endonuclease digestion of pSB1A2-PlacO-1-RBS1.0-luxI-TT-PlacO-1-RBS1.0-luxR-TT-luxpR-RBS0.3-lacZɑ-ccdB
Week 8(22nd May—28th May)
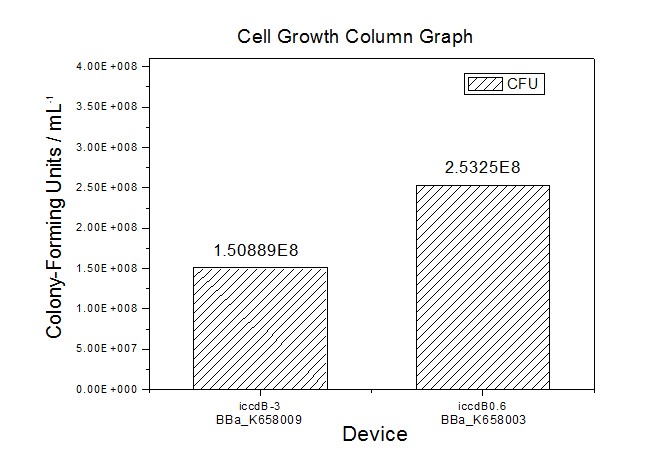
Conducting the experiment on the cell growth of the two devices with RBS0.07.

Conducting the experiment on the viable cell density at steady state of the four devices with RBS0.07, RBS0.3, RBS0.6 and RBS1.0

Week 9-17(29th May—30th Jul.)
Aim:
The promoter lux pR can also affect the functioning of the device. So we plan to mutate the promoter lux pR to see how the mutation in lux pR can affect functioning of device. Before testing on the population-control device, we try to construct another device to test how the mutation of the promoter can affect the expression of the downstream gene. In the following experiment, we plan to use the gene of gfp to show the expression.
Performance:
Week 9(29th May—4th Jun.)
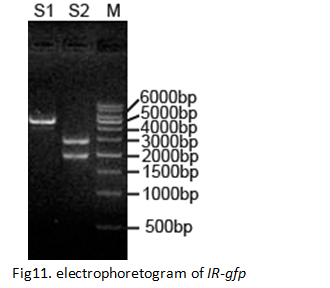
Constructing the device IR-gfp (BBa_K658016)
Week 10(5th Jun.—11th Jun.)
Experiment suspended because of exams.
Week 11(12th Jun.—18th Jun.)
Mutation of lux pR being carried out
Week 12(19th Jun. —25th Jun.)
Experiment suspended becauce of an internship in a factory away from Xiamen.
Week 13(26th Jun.—2nd Jul.)
Continuing the work of last week and getting three mutants Proving the three mutants wrong which is shown as follows:

Week 14(3rd Jul.--9th Jul.)
By restriction analysis, the restored IR-gfp identified to be broken

Reconstructing IR-gfp

Week 15-16(10th Jul.--23rd Jul.)
Successfully getting the mutant at site 3, site 5 and site 3/5 which can be verified by sequence analysis

Week 17(24th Jul.—30th Jul.)
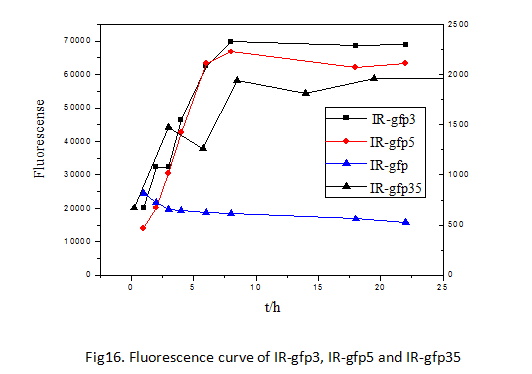
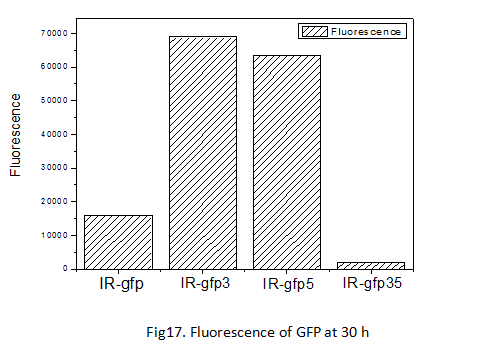
Conducting the experiment of determining the fluorescence curve of the three devices with mutations respectively at site 3, 5, 3/5

Week 18-21(31st Jul.—27th Aug.)
Aim:
We plan to mutate the lux pR to see how the mutation in lux pR can affect functioning of the population-control device.
Performance:
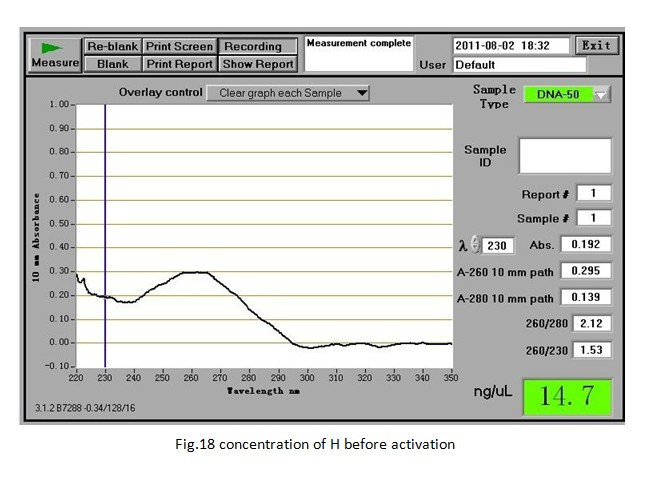
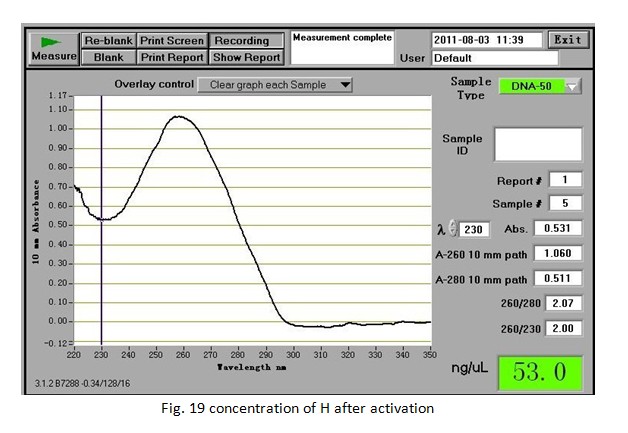
Week 16(31st Jul. —6th Aug.) Activation of H for the following experiment

Successfully getting the mutant H3
The intended 5-site mutation turning out to be mutated at 5/15 and 5/20
Week 17-18(7th Aug.—20th Aug.)
Successfully getting the mutant at site 5 and 3/5
Week 19(21st Aug. —27th Aug.)
Conducting the experiment of the bacteria quantity of the population-control device with mutations respectively at site 3, 5, 3/5

Week 20-22(28th Aug.—17th Sep.)
Aim:
In order to further study whether the population-control device has an impact on the the expression of the downstream gene, we plan to add the gene gfp to the population-control device to examine it.
Performance:
Week 20(28th Aug.—3rd Sep.)
Constructing the BioBrick H62M19(BBa_K658020)
Constructing 62M19(BBa_K658021)

Week 21(4th Sep.—10th Sep.)
Conducting the experiment of determining the fluorescence curves of H62M19 and 62M19
Reviewing the experimental process of the week and sorting out mistakes we have made
Week 22(11th Sep.—17th Sep.)
Repeating and optimizing the work done last week to get better results.

Week 23(18th Sep.—24th Sep.)
Changing the backbone of the plasmids according to the requirement
 "
"