Team:USC
From 2011.igem.org
Kevinle1992 (Talk | contribs) |
|||
| (7 intermediate revisions not shown) | |||
| Line 31: | Line 31: | ||
<tr> | <tr> | ||
<td style="width:100px; vertical-align:top;"> | <td style="width:100px; vertical-align:top;"> | ||
| - | |||
| - | |||
<td style="width:724px;"> | <td style="width:724px;"> | ||
<table> | <table> | ||
<tr> | <tr> | ||
| - | <h1 style="font-family:Verdana;font-weight:700;">E. | + | <h1 style="font-family:Verdana;font-weight:700;">E. curi</h1> |
</tr> | </tr> | ||
| Line 48: | Line 46: | ||
<tr> | <tr> | ||
| - | <td style=" text-align:center; width: | + | <td style=" text-align:center; width:724px;"> |
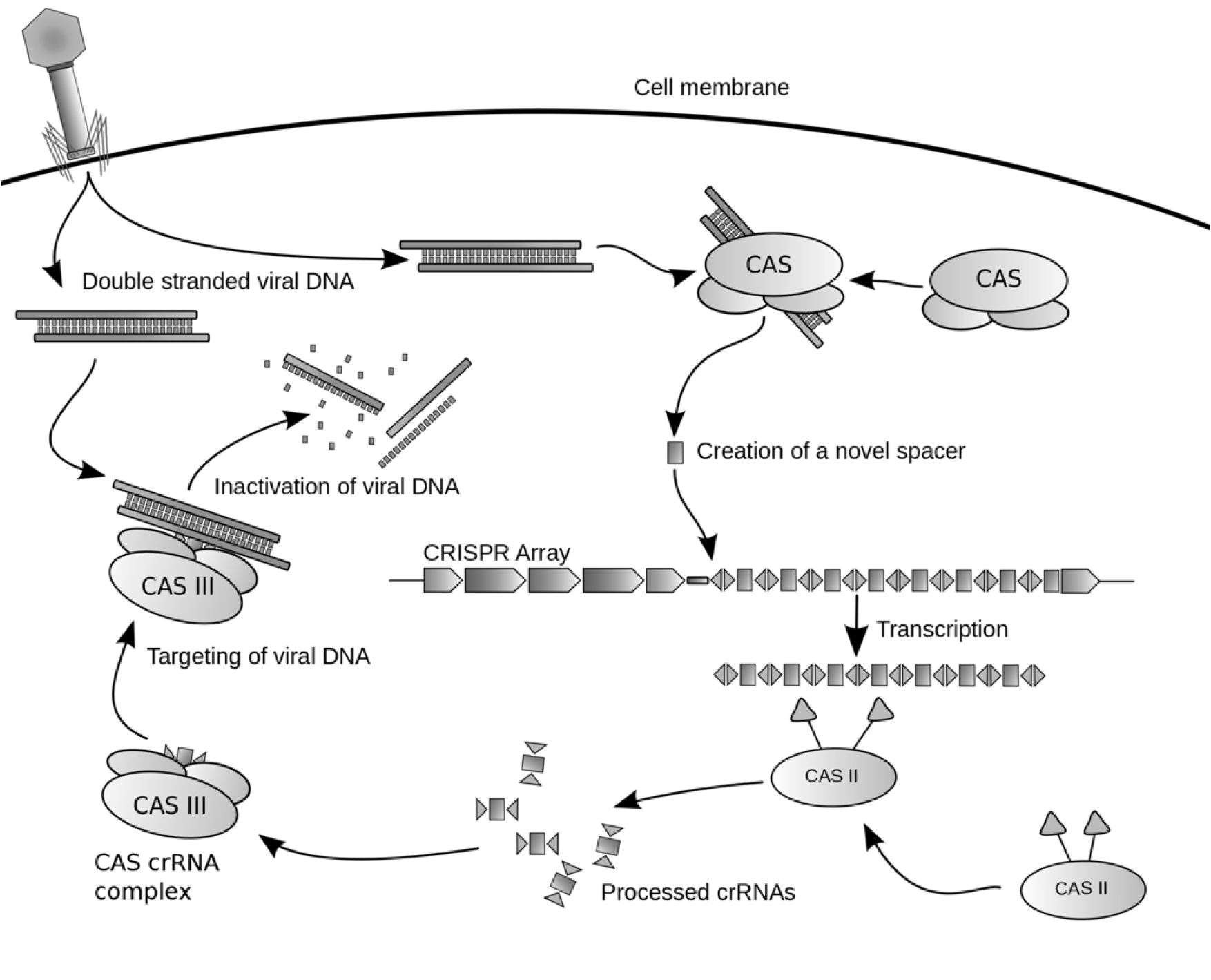
| - | [[File:CRISPRworks.png]] | + | [[File:CRISPRworks.png|600px|center]] |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 56: | Line 54: | ||
<td> | <td> | ||
<p style="font-family:Verdana;"> | <p style="font-family:Verdana;"> | ||
| - | + | We synthesized a version of CRISPR encoding a spacer that matches the GFP DNA sequence. We tested the synthetic CRISPR array against E.coli harboring a tetO::GFP plasmid that confers ampicillin resistance. Activation of CRISPER-GFP destroys the GFP-containing plasmid restoring the bacterial host's sensitivity to ampicillin. We will use the synthetic CRISPR system as a biological tool, combining it with other BioBricks for use in applications that will impact health and medicine, biotechnology, molecular biology, and genetics. | |
</p> | </p> | ||
</td> | </td> | ||
</tr> | </tr> | ||
| + | <tr> | ||
| + | <td style="width:100px; vertical-align:top;"> | ||
| + | |||
| + | <h1 style="font-family:Verdana;font-weight:700;">Our Sponsors</h1> | ||
| + | {|border="1px" align="center" style="text-align:center;" | ||
| + | |[[File:curranlab.jpg | 150px | ]] || Curran Laboratory at the USC School of Gerontology | ||
| + | |- | ||
| + | |[[File:Labofmicriobio wageningen.gif | 400px | ]] || Brouns Laboratory at the University of Wageningen | ||
| + | |- | ||
| + | |[[File:IDTLogo2010.png | 200px | ]] || Integrated DNA Technologies | ||
| + | |- | ||
| + | |[[File:dornsife.jpg | 100px | ]] || David and Dana Dornsife College of Arts and Sciences | ||
| + | |- | ||
| + | |[[File:viterbi.gif | 200px | ]] || Viterbi School of Engineering | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | <td style="width:100px; vertical-align:top;"> | ||
| + | |||
| + | </td> | ||
| + | </tr> | ||
</table> | </table> | ||
</td> | </td> | ||
</tr> | </tr> | ||
</table> | </table> | ||
Latest revision as of 04:11, 29 September 2011
 |
||||||||||||||||
|
|
||||||||||||||||
|
||||||||||||||||
 "
"