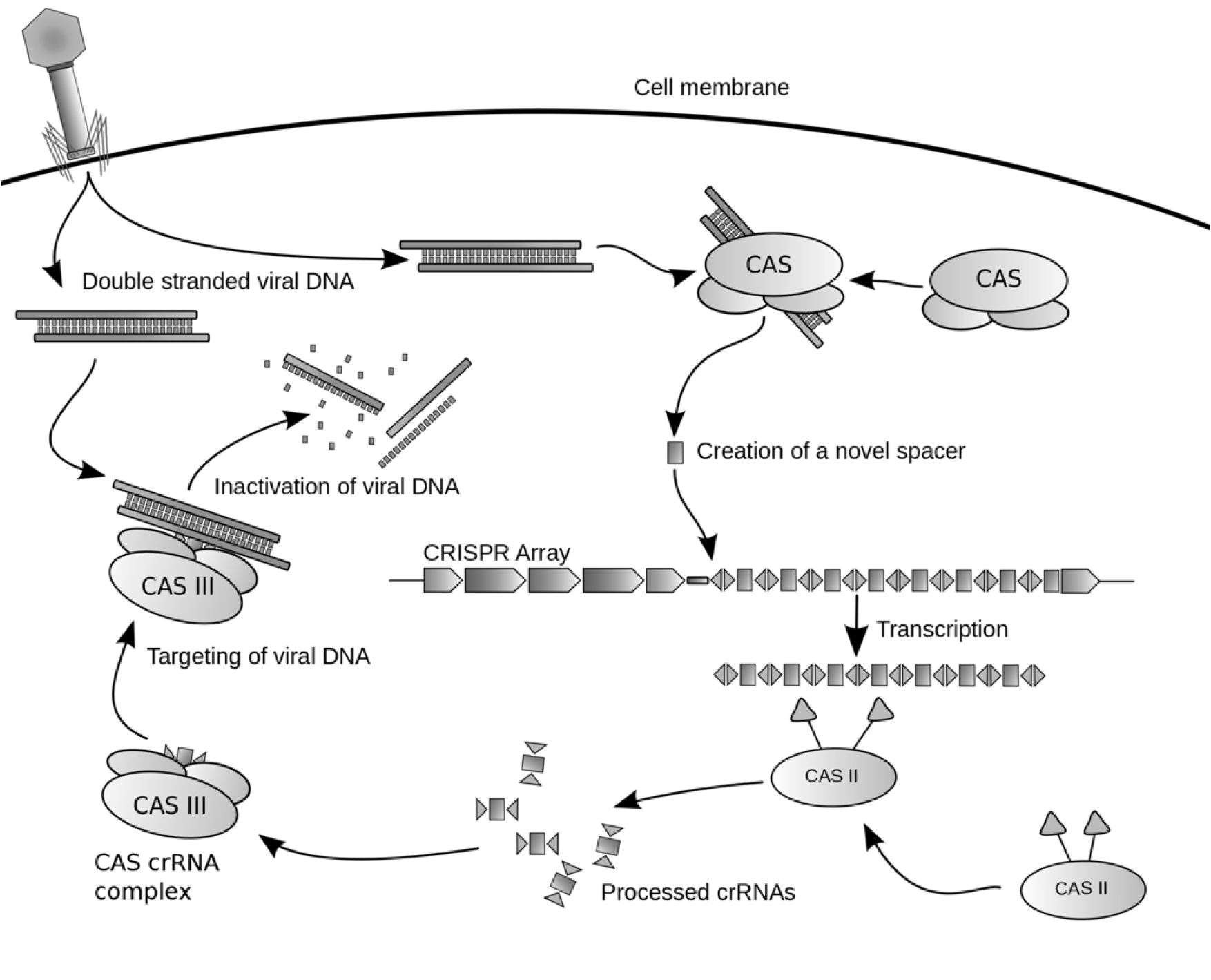
Team:USC
From 2011.igem.org
Kevinle1992 (Talk | contribs) |
Kevinle1992 (Talk | contribs) |
||
| Line 66: | Line 66: | ||
|[[File:curranlab.jpg | 200px | ]] || Curran Laboratory at the University of Southern California | |[[File:curranlab.jpg | 200px | ]] || Curran Laboratory at the University of Southern California | ||
|- | |- | ||
| - | |[[File:Labofmicriobio wageningen.gif | | + | |[[File:Labofmicriobio wageningen.gif | 300px | ]] || Brouns Laboratory at the University of Wageningen |
|- | |- | ||
| - | |[[File: | + | |[[File:IDTLogo2010.png | 200px | ]] || Integrated DNA Technologies |
|- | |- | ||
|[[File:dornsife.jpg | 100px | ]] || David and Dana Dornsife College of Arts and Sciences | |[[File:dornsife.jpg | 100px | ]] || David and Dana Dornsife College of Arts and Sciences | ||
|- | |- | ||
| - | |[[File:viterbi.gif | | + | |[[File:viterbi.gif | 200px | ]] || Viterbi School of Engineering |
|- | |- | ||
|} | |} | ||
Revision as of 00:58, 29 September 2011
 |
||||||||||||||||
|
|
||||||||||||||||
|
||||||||||||||||
 "
"