Team:UEA-JIC Norwich/Nittygritty-bacteria
From 2011.igem.org
Making bacteria see.
We are planning to transform the bacterial species Escherichia coli. We chose E.coli because its genome has been completely sequenced, it has a high transformation frequency and is relatively simple to manipulate. As most iGEM projects have centered around E.coli most Biobricks are also optimised for expression in this species. We therefore wished to first use E.coli to test that the luminescence could be achieved and that a form of control was also possible, before we moved onto the more advanced eukaryotic species we planned to work with. We plan to ultimately transform E.coli with a new composite Biobrick. This will be formed of four existing Biobricks including Cph8 (BBa_I15010), OmpR (BBa_R0082), Green Luciferase (BBa_K325229) and Luciferin Recovery Enzyme (BBa_K325202).
Cph8 is a composite part formed of the Cph1 and Env Biobricks. Cph1 is a light sensing domain most sensitive to red light in the region of 660nm. When translated, light exposure inhibits the activity of the EnvZ histidine kinase domain. Thus, the kinase activity will only be active in the dark. When in this state, EnvZ phosphorylates the OmpR promoter to OmpR-P. This promoter is usually upstream of the OmpC gene. We are planning to place this promoter upstream of the luciferase and luciferase recovery enzyme Biobricks. Therefore, our cells will only luminesce in the dark!
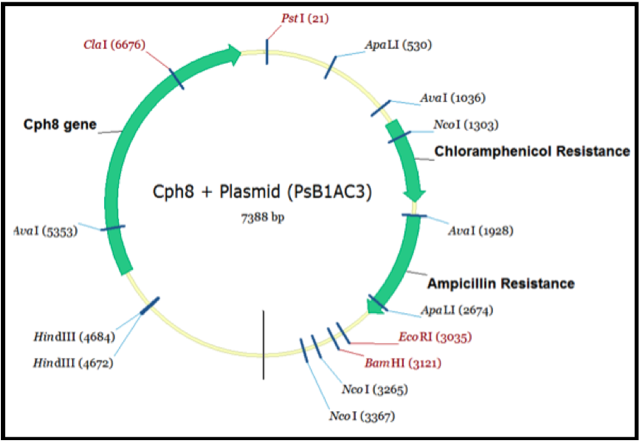
Biobrick assembly
In order to better undersand the Biobricks we were attempting to extract and combine into our new system we wished to visualise them. We entered the nucleotide sequences into the program Vector NTI. This allowed as to locate the restriction sites and highlight the areas of the sequence containing the genes we desired. We then used the information to create a view of the Biobrick we ultimately wished to create.
Experimental Plan
Week 1 & 2
1. Conduct transformations of all desired biobricks: OmpR (BBa_r0082), Cph8 (BBa_i15010), Green Luciferase (BBa_k325229), Luciferin Regnerating Enzyme (BBa_k325202) and Green Luciferase and LRE composite (BBa_i1308)
Week 3
1. Make culture and glycerol stock solutions of above successful Biobricks (OmpR, Green Luciferase and Luciferin Regenerating Enzyme)
2. Make further transformation of the Biobrick BBa_M30109 (this contains an alternative source of Cph8)
3. Do mini prep plasmid extraction of above transformed E.coli cells
Week 4
1. PCR of Cph8
2. Run gels to ensure successful gene extraction, and restriction digest to ensure fragments of the correct size are present
3. Insert into plasmid
4. Do ligation of Biobricks Cph8, OmpR, Green Luciferase and Luciferin Recovery Enzyme and insert into the plasmid
5. Test Green Luciferase with Luciferin substrate
Week 5
1. Run gel of ligated biobrick and restriction digest gel to ensure correct size and fragment size
2. Transformation of the composite biobrick formed of the genes into E.Coli
3. Make culture and glycerol stock solution of above transformed cells
4. Light detection following administration of Luciferin.
 "
"