Team:Missouri Miners/Data
From 2011.igem.org
(Difference between revisions)
| Line 11: | Line 11: | ||
| - | + | <h3>Sequence Verification</h3><br/> | |
This part has been sequence verified and works as expected. <br/><br/> | This part has been sequence verified and works as expected. <br/><br/> | ||
| - | + | <h3>Fluorescence wavelengths</h3><br/> | |
*'''Excitation max''' - 485nm<br/> | *'''Excitation max''' - 485nm<br/> | ||
*'''Emission max''' - 520nm<br/><br/> | *'''Emission max''' - 520nm<br/><br/> | ||
| Line 21: | Line 21: | ||
| - | + | <h3>Growth Conditions</h3><br/> | |
*'''Growth Temperature and Rate''' - E. colistrain DH5alphaused as Chassis, standard growth conditions apply <br/> | *'''Growth Temperature and Rate''' - E. colistrain DH5alphaused as Chassis, standard growth conditions apply <br/> | ||
*'''Growth Media''' - Nutrient Broth or diluted LB media, since OmpR is an osmolarity receptor the cells must be grown in a low osmolarity media to control for compounding factors. To express Fluorescence it is recommended that cultures are grown in liquid media. Colonies growing on plates cannot detect osmolarity and will not activate EnvZ. <br/> | *'''Growth Media''' - Nutrient Broth or diluted LB media, since OmpR is an osmolarity receptor the cells must be grown in a low osmolarity media to control for compounding factors. To express Fluorescence it is recommended that cultures are grown in liquid media. Colonies growing on plates cannot detect osmolarity and will not activate EnvZ. <br/> | ||
| Line 31: | Line 31: | ||
<br/><br/> | <br/><br/> | ||
| - | + | <h3>Dose Response</h3><br/> | |
To control for compounding variables, the cells assayed were grown in 0.5X LB media. This reduced the background osmolarity and allowed our team to measure a valid response. In the graph you can see that the system fluoresces over background at 1% Glucose and then drops back to background at 5% Glucose. The increase at 1% is due to positive regulation by the EnvZ-OmpR system. 5% glucose is a high enough concentration to induce downregulation of the system. Too many phosphorylated OmpR proteins bind the ompC promoter. When all three binding sites are filled RNAP cannot transcribe downstream and fluorescence decreases. <br/><br/> | To control for compounding variables, the cells assayed were grown in 0.5X LB media. This reduced the background osmolarity and allowed our team to measure a valid response. In the graph you can see that the system fluoresces over background at 1% Glucose and then drops back to background at 5% Glucose. The increase at 1% is due to positive regulation by the EnvZ-OmpR system. 5% glucose is a high enough concentration to induce downregulation of the system. Too many phosphorylated OmpR proteins bind the ompC promoter. When all three binding sites are filled RNAP cannot transcribe downstream and fluorescence decreases. <br/><br/> | ||
| Line 37: | Line 37: | ||
<img src="https://static.igem.org/mediawiki/2011/4/48/Dose_response_1.jpg" alt="Dose Response"> <br/><br/> | <img src="https://static.igem.org/mediawiki/2011/4/48/Dose_response_1.jpg" alt="Dose Response"> <br/><br/> | ||
| - | + | <h3>Time Trial</h3><br/> | |
The graph below shows time trials of the system. These time trials were run in the presence 0%, 1%, and 8% glucose. We chose to do this so we could gather more comprehensive data. The first conclusion we can draw from this graph is that the response is fairly consistent over time (there is no peak in fluorescence associated with a specific time point). Also, for each time point between 0.5-4.5 hrs, the curve of the dose response remains consistent. This graph also shows that the assay is not accurate after 4.5 hours. The data beyond 4.5 hours becomes random and inconsistent.<br/><br/> | The graph below shows time trials of the system. These time trials were run in the presence 0%, 1%, and 8% glucose. We chose to do this so we could gather more comprehensive data. The first conclusion we can draw from this graph is that the response is fairly consistent over time (there is no peak in fluorescence associated with a specific time point). Also, for each time point between 0.5-4.5 hrs, the curve of the dose response remains consistent. This graph also shows that the assay is not accurate after 4.5 hours. The data beyond 4.5 hours becomes random and inconsistent.<br/><br/> | ||
| - | <img src="https://static.igem.org/mediawiki/2011/f/fe/Time_Trials_2.jpg" alt="Time Trials"><br/><br/> | + | <img src="https://static.igem.org/mediawiki/2011/f/fe/Time_Trials_2.jpg" alt="Time Trials" align="top"><br/><br/> |
</div> | </div> | ||
</html> | </html> | ||
Revision as of 03:18, 29 September 2011
Data
Our part consists of an OmpR binding region that regulates our reporter gene, eyfp. The OmpR binding region is the ompC operator, which has three OmpR binding sites. Our part is regulated by the EnvZ-OmpR two-component regulatory system. EnzZ is aosmolarity sensor on the inner membrane and phosphorylates OmpR in the presence of high osmolarity. When phosphorylated, OmpR binds to the ompC operator and recruits RNAP to transcribe the downstream reporter gene. Fluorescence intensity due to the production of eYfp is a measure of the system's activity. When all three binding sites are occupied by phosphorylated OmpR proteins, RNAP cannot bind to the DNA and transcribe downstream. This part can be used as an osmolarity indicator. Our team used this part as a glucose sensor because the presence of glucose changes osmolarity. Different concentrations of glucose produce different, quantifiable fluorescence intensities.Sequence Verification
This part has been sequence verified and works as expected.
Fluorescence wavelengths
*'''Excitation max''' - 485nm
*'''Emission max''' - 520nm
Growth Conditions
*'''Growth Temperature and Rate''' - E. colistrain DH5alphaused as Chassis, standard growth conditions apply
*'''Growth Media''' - Nutrient Broth or diluted LB media, since OmpR is an osmolarity receptor the cells must be grown in a low osmolarity media to control for compounding factors. To express Fluorescence it is recommended that cultures are grown in liquid media. Colonies growing on plates cannot detect osmolarity and will not activate EnvZ.
If cells are grown in a high osmolarity media, such as LB broth, the background fluorescence will be higher than an induced glucose response. This is because the system is already primed from the high osmolarity of the media. When additional osmolites are added, the system will down regulate eyfp due to the presence of too many phosphorylated OmpR proteins. Below is a dose response in the presence of LB media. Note how glucose, in these conditions, has an inhibitory effect on the system.
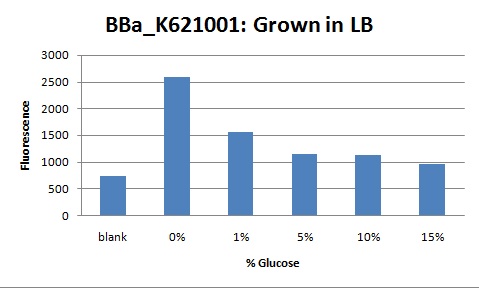
Dose Response
To control for compounding variables, the cells assayed were grown in 0.5X LB media. This reduced the background osmolarity and allowed our team to measure a valid response. In the graph you can see that the system fluoresces over background at 1% Glucose and then drops back to background at 5% Glucose. The increase at 1% is due to positive regulation by the EnvZ-OmpR system. 5% glucose is a high enough concentration to induce downregulation of the system. Too many phosphorylated OmpR proteins bind the ompC promoter. When all three binding sites are filled RNAP cannot transcribe downstream and fluorescence decreases.

Time Trial
The graph below shows time trials of the system. These time trials were run in the presence 0%, 1%, and 8% glucose. We chose to do this so we could gather more comprehensive data. The first conclusion we can draw from this graph is that the response is fairly consistent over time (there is no peak in fluorescence associated with a specific time point). Also, for each time point between 0.5-4.5 hrs, the curve of the dose response remains consistent. This graph also shows that the assay is not accurate after 4.5 hours. The data beyond 4.5 hours becomes random and inconsistent.

 "
"