Overview
Here you can see what we did weekly in the lab!
Week 1: June 6 - June 11
In the first week we began constructing basic reporter DNA constructs that have various combinations of promoters and genes. The mix-and-matching of promoters and genes was done using the LR reaction following the Gateway protocol. Our promoters included TRE, minCMV, Hef1a, Hefl1a-LacO, and our genes included eYFP, mKate, and eBFP2. Transformed and inoculated colonies from the LR reactions and performed restriction digests. Approximately half of the LR reactions were successful and became DNA parts that we could use later on.
June 7, 2011
Date
|
Assignee
|
DEST_R4R2
|
L4-R1 Promoter
|
L1-L2
Gene
|
LR?
|
Tube Number
|
Colony Number,
Antibiotic, Person
|
Protocol Used |
6.7.2011
|
Michelle
Louis
|
2-3
|
Tre
|
C1434VP16
|
Yes. 6.7.2011, 5pm
|
11
|
5, AMP, KH
|
Standard, 30C for 16h
|
6.7.2011
|
Michelle
Louis
|
2-3
|
Tre
|
Mnt1VP16
|
Yes. 6.7.2011, 5pm
|
12
|
4, AMP, KH |
Standard, 30C for 16h |
6.7.2011
|
Tyler
|
2-3
|
Tre
|
LacIKrab
|
Yes. 6.7.2011, 5pm
|
6
|
30, AMP, KH |
Standard, 30C for 16h |
6.7.2011
|
Jenny
Divya
|
3-4
|
minCMV-7xMnt1
|
eYFP
|
Yes. 6.7.2011, 5pm
|
9
|
0, AMP, KH, needs to be redone
|
Standard, 30C for 16h |
6.7.2011
|
Jenny
Divya
|
2-3
|
Tre
|
LexAVP16
|
Yes. 6.7.2011, 5pm
|
10
|
2, AMP, KH, needs to be redone
|
Standard, 30C for 16h |
| 6.7.2011 |
Mariola
Kenneth
|
3-4
|
minCMV 1xC1434
|
eYFP
|
Yes. 6.7.2011, 5pm
|
7
|
3, AMP, KH, needs to be redone |
Standard, 30C for 16h |
| 6.7.2011 |
Mariola
Kenneth |
3-4 |
minCMV 4xLexA
|
eYFP
|
Yes. 6.7.2011, 5pm
|
8
|
0, AMP, KH, needs to be redone
|
Standard, 30C for 16h |
LR Reactions
Utilizing protocol here: LR Protocol
Date
|
Assignee
|
DEST_R4R2
|
L4-R1 Promoter
|
L1-L2
Gene
|
LR?
|
Tube Number
|
Colony Number,
Antibiotic, Person
|
Protocol Used |
6.9.2011
|
Grant
|
3-4
|
minCMV 4xLexA |
eYFP |
Yes. 6.9.2011, 3pm
|
12
|
10, AMP, GR
|
Standard + Grown at 37C in LB not SOC. |
6.9.2011
|
Jon C
|
3-4
|
minCMV-4xMnt1
|
eYFP
|
Yes. 6.9.2011, 3:45pm |
2
|
13, AMP, GR
|
Standard + Grown at 37C in LB not SOC. |
6.9.2011
|
Jon C
|
3-4
|
minCMV-4xCI434
|
eYFP
|
Yes. 6.9.2011, 3:45pm |
1
|
7, AMP, GR |
Standard + Grown at 37C in LB not SOC. |
6.9.2011
|
Jon C
|
3-4
|
Hef1a-LacO
|
eYFP
|
Yes, 6.9.2011, 4:20pm
|
5
|
25, AMP, GR
|
Standard + Grown at 37C in LB not SOC. |
6.9.2011
|
Semon
|
4-5
|
Tre
|
mKate
|
Yes, 6.9.2011 4:27pm
|
3
|
6, AMP, GR
|
Standard + Grown at 37C in LB not SOC. |
6.9.2011
|
Semon
|
1-2
|
Hef1a
|
eBFP2
|
Yes, 6.9.2011, 4.27pm
|
4
|
3, AMP, GR
|
Standard + Grown at 37C in LB not SOC. |
Transformation
Date
|
Assignee
|
DEST_R4R2
|
L4-R1 Promoter
|
L1-L2
Gene
|
LR?
|
|
Colony Number,
Antibiotic
|
Protocol Used |
6.9.2011
|
Mariola
|
|
|
eYFP
|
no. Replication stock. |
|
2, AMP
|
Transformation (~ 2 hr 20 min) using LB instead of SOC |
Miniprep
| Date |
Assignee
|
DNA
|
Quantity
|
Time collected
|
6.9.2011
|
Divya-Jenny
|
pEXPR_2-3_Tre:LexAVP16
|
52.2 ng/uL
|
12pm
|
6.9.2011
|
Kenneth
|
pEXPR_3-4_minCMV-CI434:eYFP (A)
|
70 ng/ul
|
12pm
|
6.9.2011
|
Kenneth |
pEXPR_3-4_minCMV-CI434:eYFP (B)
|
174 ng/uL
|
12pm
|
6.9.2011
|
Louis
|
pEXPR_2-3_Tre:C1434VP16
|
234.6 ng/uL
|
12pm
|
6.9.2011
|
Tyler
|
pEXPR_2-3_Tre:Lac/Krab
|
130.7 ng/uL
|
12pm
|
6.9.2011
|
Michelle
|
pEXPR_2-3_Tre:Mnt1VP16
|
110.0 ng/uL
|
12pm
|
6.9.2011
|
Michelle
|
pDEST_2-3_ccdB
|
117.6 ng/uL
|
12pm
|
Restriction Digests
Assignee
|
DNA
|
Enzyme
|
Expected Results
|
Picture of Gel
|
Time Incubated |
Comments
|
Louis
|
pEXPR_2-3_Tre:C1434VP16 |
NdeI
|
6700 bp
800 bp |
a.3 |
6/9/11
3:50 PM |
|
|
|
Kenneth
|
pEXPR_3-4_minCMV-CI434:eYFP (A+B)
|
NcoI and SacII
|
2450 bp
4650 bp
|
A: a.6
B: a.7
|
6/9/11
3:00 PM
|
A did not cut as expected. B looks good.
Digestion Mix: 2 uL NEB4, 0.5 uL of each enzyme, A: 7.1 uL/B: 2.9 uL of DNA, 10.4 uL/14.6 uL of H20
|
|
|
Michelle
|
pEXPR_2-3_Tre:Mnt1VP16
|
BglI
|
3700 bp
2200 bp
1300 bp
|
a.1 |
6/9/11
4:00PM
|
DNA appeared to be uncut. Need to redo, possibly with a new enzyme and more DNA.
|
|
|
Michelle
|
pDEST_2-3_ccdB
|
NcoI and NheI
|
6100 bp
1700 bp
|
a.2 |
6/9/2011
4:25 PM
|
Attained bands at correct positions. Other band represents partially cut DNA in double digest.
|
|
|
Divya
|
pEXPR_2-3_Tre:LexAVP16
|
HincII
|
|
a.4 |
6/9/2011
4:45 PM
|
- Bands didn't show up. Needs to be redone.
- DNA may have degraded. Need to redo Nanodrop as well. |
|
|
| Tyler |
pEXPR_2-3_Tre:Lac/Krab |
SalI |
3 bands:
5288 bp
1510 bp
1241 bp
cuts gene |
a.5 |
6/9/2011
3:00 PM |
Digestion:
4 uL * 130.7 ng/uL = 522.8 ng DNA
2 uL NEB3
2 uL BSA
11 uL H2O
1 uL SalI
Total: 20 uL
Only saw one band at 3500 bp
Needs to be redone possibly with another restriction enzyme |
|
|
Gels
| Label |
Picture |
| a |
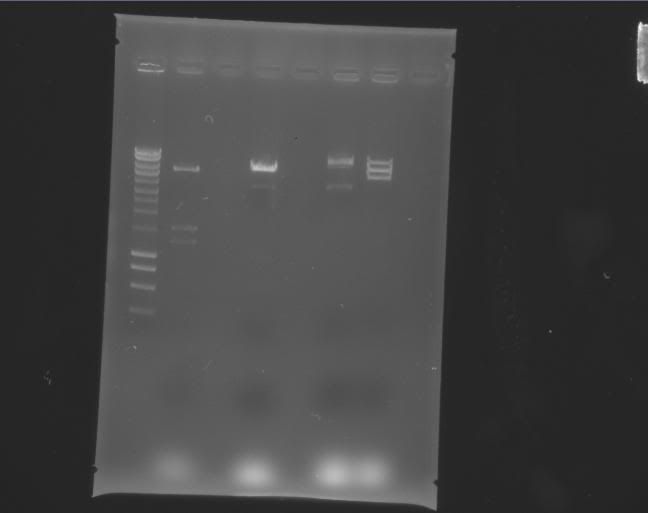
|
June 10, 2011
Restriction Gels
| Assignee |
DNA
|
Enzyme
|
Expected result
|
Time Incubated
|
Comments
|
Louis
|
pEXPR_2-3_Tre:C1434VP16 |
EcoRI (Buffer 2)
|
5500 bp
1050 bp
650 bp
360 bp |
6/10/11
11:15 AM |
|
Michelle
|
pEXPR_2-3_TRE:Mnt1VP16 (A)
|
NcoI and NheI
|
5700 bp
1300 bp
|
6/10/11
11:40 AM
|
Digestion:
6 uL DNA
1 uL NcoI
1 uL Nhe1
2 uL NEB2
2 uL BSA
8 uL H2O
Failed.
|
Michelle
|
pEXPR_2-3_TRE:Mnt1VP16 (B)
|
NheI and SphI
|
1700 bp
5300 bp
|
6/10/11
11:40 AM
|
Digestion:
6 uL DNA
1 uL NheI
1 uL SphI
2 uL NEB2
2 uL BSA
8 uL H2O
Failed. Redoing LR on Monday June 13th.
|
| Tyler |
pEXPR_2-3_Tre:Lac/Krab |
SalI |
3 bands:
5288 bp
1510 bp
1241 bp
cuts gene |
6/10/2011
3:00 PM |
Digestion: 4 uL * 130.7 ng/uL = 522.8 ng DNA
2 uL NEB3
2 uL BSA
11 uL H2O
1 uL SalI
Total: 20 uL
***Worked (see gel below - b.1)
Stored in -80C freezer |
Comments:
pEXPR_2-3_Tre:LexAVP16 and pEXPR_3-4_minCMV-7xMnt1:eYFP are being entirely redone.
pEXPR_2-3_Tre:LexAVP16 (post-miniprep) was lost. pEXPR_3-4_minCMV-7xMnt1:eYFP LR was unsuccessful (no bacteria grew).
LR and transformation will be done on Saturday. Miniprep and restriction mapping will be done on Sunday
| Label |
Gel
|
Legend
|
b
|
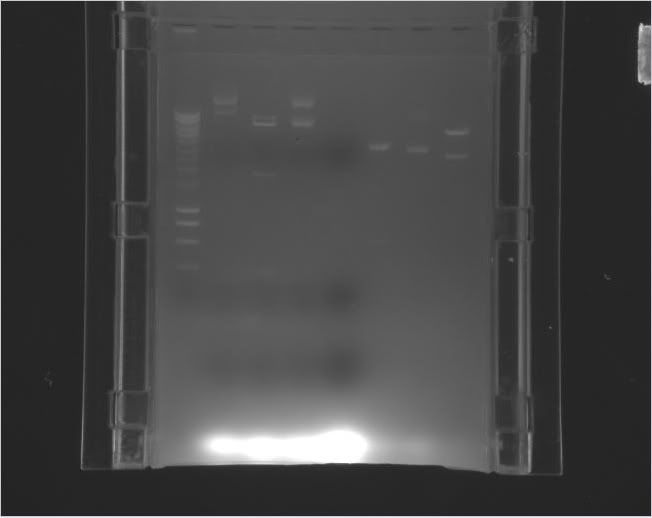
|
Column 0: HyperLadder
Column 1: pEXPR_2-3_Tre:Lac/Krab
Column 4: pEXPR_2-3_Tre:C1434VP16
Column 6: pEXPR_2-3_TRE:Mnt1VP16 (A)
Column 7: pEXPR_2-3_TRE:Mnt1VP16 (B) |
June 11, 2011
Set of LRs from 6/9 (GR, JC, and SR) cell counts taken; inoculated by GR/JC. 3 cells taken per plate.
Assignee
|
DNA
|
Enzyme
|
Expected Results
|
Time Incubated
|
Comments
|
Sam
|
pEXPR_1-2_Hef:eBFP
|
NcoI & ApaL1
|
3750bp, 3180bp, 1250bp |
45 min |
If it doesn't work there should be a whole mess of fragments (including a small 600bp one)
1mL each enz; 2 mL Buf; 1 mL BSA; 2mL DNA; 13mL H2O (Standard Protocol with extra BSA)
|
Sam
|
pEXPR_4-5_Tre:mKate
|
Bgl1
|
1270bp, 2630bp, 3380bp |
45 min |
1mL enz; 2mL DNA; 2mL BSA; 2mL Buf; 15mL H2O (Standard Protocol with extra BSA and water - not quite 10x)
|
Week 2: June 12 - June 18
In the second week we attempted midipreps instead of minipreps of our available cell stocks. Midipreps were unsuccessful, likely due to centrifuge limitations. LR reactions to generate more usable promoter-gene pair parts continued, expanding to include the CI434-VP16, LexA-VP16, and Mnt-VP16 genes, whose expressed proteins activate the appropriate corresponding minCMV promoters. Most of the LRs done were not successful, reason unknown at this time.
June 13, 2011
Status
| DNA |
Status
|
Comment
|
Mntcmv/1x cI434:eYFP
|
good
|
|
Mnt cmv/4x LexA:eYFP
|
bad |
|
HefIa/LacO:eYFP
|
good |
|
Tre:LacI Krab
|
good |
|
HefIaL EBFP2
|
bad |
|
Date
|
Assignee
|
DEST_R4R2
|
L4-R1 Promoter
|
L1-L2
Gene
|
LR?
|
Tube Number
|
Colony Number,
Antibiotic, Person
|
Protocol Used |
6.13.2011
|
Jon
|
2-3
|
Tre |
C1434-VP16 |
Yes. 6-13, 11am
|
10
|
about 50, AMP, JC
|
No |
6.13.2011
|
Tyler
|
2-3
|
Tre
|
LexA-VP16
|
Yes. 6-13, 11am |
9
|
about 30, AMP, GR
|
No |
6.13.2011
|
Mariola
|
2-3
|
Tre
|
Mnt1-VP16
|
Yes. 6-13, 11am |
Eppendorf
|
0, AMP, GR |
No |
6.13.2011
|
Semon
|
4-5
|
Tre
|
mKate
|
Yes. 6-13, 11am
|
11
|
about 10, AMP, SR
|
No |
6.13.2011
|
Divya
|
3-4
|
minCMV-7xMnt1
|
eYFP
|
Yes 6/13, 1:30pm
|
?
|
1000s, AMP, GR
|
No |
6.13.2011
|
Jenny
|
3-4
|
minCMV-4xMnt1
|
eYFP
|
Yes 6/13, 1:30pm
|
?
|
1000s, AMP, GR
|
No |
6.13.2011
|
Jenny
|
3-4
|
minCMV-4xC1434
|
eYFP
|
Yes 6/13,1:30pm
|
?
|
1000s, AMP, GR
|
No |
June 14, 2011
Highlights:
~10:30 AM: Miniprep of pDEST23-ccdB commenced and completed. DNA concentrations of the two samples were 175 and 310 ng/uL
~1:00 PM: Inoculation of cultures for pEXPR-23-TRE-CI434-VP16, pEXPR-23-TRE-LexA-VP16, pEXPR-45-TRE-mKate, pEXPR-34-minCMV-7xMnt-eYFP, pEXPR-34-minCMV-4xMnt-eYFP, and pEXPR-minCMV-4xCI434 were conducted. Colony counts from the previous transformations are noted above.
~1:30 PM: LR Gateway reactions were performed for the failed pEXPR-23-TRE-Mnt-VP16 and the new pEXPR-12-Hef1A-rtTA.
~5:00 PM: Inoculation of midiprep cultures for EXPR-34-minCMV4xLexA-eYFP, two samples of pEXPR-34-Hef1ALacO-eYFP, pEXPR-12-Hef1A-EBFP, pEXPR-23-TRE-LacIKrab, and pEXPR-34-minCMV1xcI434-eYFP commenced. We plan to midiprep the cells at about 10:00 AM.
~6:00 PM: Transformation of the aforementioned LR Gateway reactions commenced. Transformation completed around 9:00 PM.
~7:00 PM: Design for the TANGO primers complete.
June 15, 2011
~4AM: Grant did more LRs – 3-4_minCMV-4xLexA:eYFP, 1-2_Hef1a:rttA3, 1-2_Hef1a:eBFP2
~10AM: Minipreps done on the 6 LRs that succeeded from yesterday by Grant
Nanodrops
Date
|
Assignee
|
Vector
|
Concentrations (ng/uL) *denotes 260/280 below 1.8 |
6.14 11AM
|
Jon/Michelle/Clara
|
2-3_Tre:C1434-VP16
|
A: 284.0* (1.78), B: 391.3, C: 380.9, D: 205.5 |
6.14 11AM
|
Jon/Michelle/Clara
|
2-3_Tre:LexA-VP16
|
A: 361.4, B: 193.9* (1.64), C: 212.9* (1.58), D: 390.6 |
6.14 11AM
|
Jon/Michelle/Clara
|
4-5_Tre:mKate
|
A: 176.9, B: 180.3, C: 188.7, D: 208.8 |
6.14 11AM
|
Jon/Michelle/Clara
|
3-4_minCMV-4xC1434:eYFP
|
A: 173.8, B: 152.5* (1.60), C: 319.5, D: 192.5 |
6.14 11AM
|
Jon/Michelle/Clara
|
3-4_minCMV-7xMnt1:eYFP
|
A: 147.7, B: 251.5, C: 147.6, D: 178.2 |
6.14 11AM
|
Jon/Michelle/Clara
|
3-4_minCMV-4xMnt1:eYFP
|
A: 195.1, B: 241.2* (1.75), C: 193.7, D: 157.0 |
Restriction Maps, 6.14 5PM
Assignee
|
DNA
|
Enzyme
|
Expected Results
|
Comments
|
Jon
|
3-4_minCMV-4xMnt1:eYFP
|
SalI-HF
|
5288bp, 2044bp if works; 5288bp, 1600bp, 977bp if doesn't |
All: 1uL SalI, 2uL Buf 4, 2uL BSA; A,B,C: 3uL DNA, D: 4uL DNA; DI up to 20 uL
|
Jon
|
3-4_minCMV-7xMnt1:eYFP
|
SalI-HF
|
5288bp, 2257bp if works; 5288bp, 1600bp, 977bp if doesn't |
All: 1uL SalI, 2uL Buf 4, 2uL BSA; A,B,C: 3uL DNA, D: 4uL DNA; DI up to 20 uL |
Jon
|
4-5_Tre:mKate
|
SalI-HF
|
5288bp, 1995bp if works; 5288bp, 1600bp, 977bp if doesn't |
All: 1uL SalI, 2uL Buf 4, 2uL BSA; A,B,C: 3uL DNA, D: 4uL DNA; DI up to 20 uL |
Mariola
|
2-3_Tre:C1434-VP16
|
SalI-HF
|
5288, 1862, 412 bp if works; 5288bp, 1600bp, 977bp if doesn't |
All: 1uL SalI, 2uL Buf 4, 2uL BSA; A: 1.8uL DNA, B,C: 1.3uL DNA, D: 2.4uL DNA; DI up to 20 uL |
Mariola
|
3-4_minCMV-4xC1434:eYFP
|
BamHI
|
3757, 1855, 1166, 478 bp if works; no cut if doesn't |
All: 1uL BamHI, 2uL Buf 4, 2uL BSA; A 2.9uL DNA, B: 3.2uL DNA, C: 1.6uL DNA, D: 2.6uL DNA; DI up to 20 uL |
Tyler
|
2-3_Tre:LexA-VP16
|
ScaI
|
2 cuts: 4464 and 2729 bp if works; 3 cuts: 3535, 2729, 1589 if doesn't |
All: 1uL SalI, 2uL Buf 4, 2uL BSA; A: 3uL DNA, B,D: 1.5uL DNA, C: 2.5uL DNA; DI up to 20 uL
From Gel Below:
A and C show the entry vector, B failed as well, D seems to have worked. There is uncut DNA around 7000 bp, but the other bands seem correct. D will be sent for sequencing. |

Midipreps were done by Kenneth and Tyler for sequenced verified constructs, 2-3_Tre:LacI-Krab, 3-4_minCMV 1xCI434:eYFP, and 3-4_Hef1ALac0:eYFP. Unfortunately DNA yields were low, as shown in the table below, so minipreps will be performed tomorrow, June 16, 2011.
| Date |
Assignee |
Vector |
DNA Concentration (ng/uL) |
| 6.15.2011 |
Tyler |
2-3_Tre:LacI-Krab |
54 |
| 6.15.2011 |
Kenneth |
3-4_minCMV 1xCI434:eYFP |
216 |
| 6.15.2011 |
Kenneth/Tyler |
3-4_Hef1ALac0:eYFP |
failed |
June 16, 2011
~10am Minipreps
| Date |
Assignee |
Vector |
DNA Concentration (ng/uL) |
| 6.16.2011 |
Tyler |
2-3_Tre:LacI-Krab |
509.1 |
| 6.16.2011 |
Kenneth |
3-4_minCMV 1xCI434:eYFP |
89.1 |
| 6.16.2011 |
Kenneth/Tyler |
3-4_Hef1ALac0:eYFP |
385 |
June 17-18, 2011 Construction
Protocol for all Cells: Added DNA 5 minutes after removing cells from freezer. Incubated for 30 minutes. Heat shocked for 30 seconds at 42 Celsius. Incubated on ice for two minutes. Added 1 mL of SOC. Grew at 30 C for 2 hours. Plated 100 uL from total reaction on one half of plate & the rest of the reaction on the other half of plate.
Date
|
Assignee
|
DEST_R4R2
|
Colonies (low/high conc.) |
6.18.2011
|
Grant |
pEXPR12-Hef1A-eBFP2 |
Order of <10 |
6.18.2011
|
Grant |
pEXPR12-Hef1A-rtTA3 |
Order of <10 |
6.18.2011
|
Grant |
pEXPR23-TRE-CI434.VP16 |
Order of 100-1000 |
6.18.2011
|
Grant |
pEXPR23-TRE-LexA.VP16 |
Order of 100-1000 |
6.18.2011
|
Grant |
pEXPR23-TRE-Mnt.VP16 |
Order of 100-1000 |
| 6.18.2011 |
Grant
|
pEXPR34-minCMV.4xCI434-EYFP |
Order of 1000-10000 |
| 6.18.2011 |
Grant |
pEXPR34-minCMV.4xLexA-EYFP |
Order of 1000-10000 |
| 6.18.2011 |
Grant |
pEXPR34-minCMV.4xMnt-EYFP |
Order of 1000-10000 |
| 6.18.2011 |
Grant |
pEXPR34-minCMV.7xMnt-EYFP |
Order of 1000-10000 |
| 6.18.2011 |
Grant |
pEXPR45-TRE-mKate |
Order of <10 |
Conclusion: pDEST12 appears to be growing slowly in all cases, as does pDEST45. pDEST23 grows at the rate typically expected by Gateway reaction standards while pDEST34 grows at a rate much faster than these standards. Corroborated in Weiss lab?
Week 3: June 20 - June 24
In the third week we continued expanding our library of usable DNA parts by creating more combinations of genes and promoters using the LR reaction.
Week 4: June 27 - July 1
In the fourth week we implemented a color-coded box system in our -20 freezer to accommodate for the growing need of organization of the growing number of DNA parts. We began learning and using the Gibson reaction to create the gene part AVPR2-TEVs-GV16, which is expressed to produce a vasopressin receptor bound to Gal4-VP16 by a TEV sequence that can be recognized by the TEV Protease. Gibson reaction results were not great, and it appeared that our AVPR2 DNA was not of sufficient quality, so we re-PCRed the AVPR2 DNA segment.
Week 5: July 4 - July 10
We began looking into using the Goldengate assembly method, but two gene elements contained a cut site that needed to be mutated out. We performed Site-Directed Mutagenesis using the Lightning Kit in the hopes of mutating out the cut site, but results were not successful due to mishandling during the protocol, so the procedure was set to be repeated. Gibson assembly of AVPR2-TEVs-GV16 continues as we re-PCRed the AVPR2 DNA segment and re-run the entire Gibson protocol, picking 20 colonies in a determined attempt to obtain a successful result.
<>
Week 6: July 11 - July 17
In the sixth week we continued re-attemping previous failures using modified protocols or higher quality DNA in hopes of obtaining successful reactions. We also began looking at Cadherins and the possibility of using them as a clumping mechanism for mammalian cells. In order to visualize Cadherins, we needed some sort of fluorescent color, so construction of NCadherin-EGFP (NCadherin is one of many types of cadherin) began. DNA for NCad-EGFP was ordered, but needed to be in a format such that we could LR react it with the different promoters we have. This is done by attaching attB sites to flank the NCad-EGFP gene and then performing the BP reaction, which generates LR reaction-compatible parts. PCR of the attB sites was successful. By this week we have also begun work with mammalian cell cultures. To explore the limitless possibilities of synthetic biology, a few of us took it upon themselves to look into other interesting gene components, such as the Caspase gene and the FF4 tag.
Week 7: July 18 - July 24
Part of the team worked on creating protocols for a robot liquid handler to run the usual lab reactions that we run. We are hoping that the robot can replace us and do our liquid-related lab work for us. Various dry test runs were done. We transfected various DNA parts that we have into Hek293 cells, and results show that most of our DNA works. We investigated the TRE-rtTA3 system as well as the UAS-Gal4 system. Both seemed to be functional.
Week 8: July 25 - July 31
This week we underwent a momentous drive to create more LRs. A large list of promoter-gene pairs was conceived of and we began to run through LR reactions in somewhat of a factory manner. This occupied much of our time. We also received and prepared DNA parts from Elowitz's group in Caltech. We also got trained on using the FACS machine and began to get quantitative data on our transfections.
Week 9: August 1 - August 7
In the ninth week we did a lot of internal re-organization to increase our overall efficiency in work. This involved re-organizing an internal wiki that we use for management of our available DNA as well as a log of transfections needed to be done. Lots of samples were FACS-ed, generating lots of results that we can make graphs out of. Many of our parts were successfully characterized.
Week 10: August 8 - August 14
Computer simulations of the Notch-Delta interactions were presented in our group this week, and we became convinced of the possibility of creating self-patterning mammalian cells. On the DNA side of things, we are trying to create more and more DNA using miniprep, because midipreps have for some reason not been successful for us or the 2010 iGEM team. On the transfection side, a lot of new DNA parts were transfected, observed under the microscope, and FACS-ed to quantify their effect/behavior.
Week 10
h3. August 14
Charles - Miniprepped propagations of various things, good CP LRs, as well as re-inoculations of poor CP LRs. Nanodrops were all over hte place. Some of the re-inoculated CP LRs reached 500 ng/uL, which was weird.
Divya - Ran FACS on 50 samples for Tyler and Ken. Also, I now have Koch access\! Woohoo\!\!\!
Kenneth - Prepped FACS samples. Moved frozen cells to ln2
Grant - Today and yesterday did sequencing of Tango and other plasmids as well as propagations of a handful of failed plasmids / spinning down of cells for Charles' miniprep.
h3. August 13
Kenneth - Added delta to cells. Co-cultured AVPR and pDisplay Vasopressin cells. Froze down and passed the Elowitz CHO cell lines and put in \-80. Also passed wells and flasks.
h3. August 12
Kenneth - Transfected cells for AVPR2 experiment as well as another free delta induction experiment, this time with const. color gating.
Charles - Attended Ken's orientation session and did 1 transfection just to try things out. Also obtained my new culture of cells. Cell stocked CP LR 4,5,6,7,8,11,12, of which 4,5, and 7 are tentative (repeat submitted). Re-inoculated failed CP LR's 1,2,3,9,10 with a different colony. Also propagated a whole bunch of plasmids, including the successful and tentatively successful CP LR's. Hopefully the color palette will be complete by Tuesday.
Jon - Tissue culture orientation & inoculated SDM transformations from yesterday. Colony counts around 20 for TEV and 3 for GV16.
h3. August 11
Jon - Redid GV16 and TEV SDM with new primers; transformed, hopefully they work this time.
Clara: Miniprepped and nanodropped 12 LRs from Aug 9. Hef1a: Delta-mCherry has esp. low concentration. To see conc. of other samples, refer to 8/11 [iGEM2011:Clara's Notebook]
Tyler: Prepared cells for transfection tomorrow. Plan on doing the following experiments: NCAD phenotype, TRE:LacI and TRE:LacI-Krab with proper gating, Hef1A:GV16 with proper gating, DRD2 repeat, and possible CCL5 experiment. Did FACS on [iGEM2011:Confluency Experiment] which shows best starting value around 1.5*10^5 cells/mL starting concentration. FACS machine showed around 40% efficiency. Matlab shows around 30%. In either case, we should start transfections with cells less confluent than we have been. TRE:LacI-Krab experiment seems to have worked, but there is only about 2x repression (see bottom of [Tri-Color Experiment v2|iGEM2011:Tri-Color Experiment v2]). Also put up FACS from AmCyan which is more green than blue.
Michelle: Prepped Clara's 12 minipreps for sequencing. Worked on Circuit Diagrams and write ups for the public wiki.
Charles: 42 FACS in less than 1 hour. Very fast because Ken's samples were concentrated.
h3. August 10
Tyler: I updated the wiki (first (per usual(winning))). Prepared for FACS. Transfected Confluency Experiment ([iGEM2011:Confluency Experiment]). Will FACS tomorrow. Still need FACS data from Divya for Tre:LacI-Krab experiment and some AmCyan colors. Today Jenny FACSed some DRD2 (apparently a failure from pictures) and some Delta-Notch stuff (also a failure). Need to look at the FACS, which apparently disappeared today? so hopefully we can find that tomorrow as well. Will try DRD2 (+100 uM dopamine) again later in the week with possibly more dopamine.
Charles: Completed Tyler's Hef1a_GV16 page with pretty FACS images. Watched Jenny do FACS at Koch. Some problems with either no cells in sample or visibly clumped cells. Introducing a new page... [iGEM2011:Color Palette]
Michelle: Inoculated Clara's LRs. Ready to miniprep and send for sequencing tomorrow.
Grant: Miniprepped, restriction mapped, and sequenced the minCMV promoters and a few other genes/promoters. Started LR reactions for a few more Tango parts and the Ephrin parts. Shockingly did nothing else.
h3. August 9
Kenneth: Worked on MATLAB stuff more. Treated TRE:mKate and TRE:eBFP2 experiments with dox. Transfected for pDisplay immunofluoresence experiment again. Thawed out Elowitz cell lines. Froze down large numbers of HEK and CHO cells. Passed cells into 60 mm plates for N-cad knockdown experiment. FACS data analysis. Prepared dopamine aliquots and supplies for future experiments.
Grant: Removed propagations of various gene/promoter entry vectors and inoculated a single colony from each. Did alignments of sequencing data for samples sent out yesterday. One LR reaction failed unexpectedly, but the other failures involving minCMV promoters appear to have occurred due to contamination from a Hef1a variant promoter based on a BLAST of the sequencing results. (READ: The "minCMV" promoter DNA is not actually minCMV promoter DNA\- at least this is the case for the tubes without stickers.) All parts currently being used for experiments are verified. On track for \~15-20 new LR reactions for Wednesday afternoon.
Clara: 12 LR reactions, left at room temperature @ 12:30pm~. Refer to [iGEM2011:Clara's Notebook] for details.
Charles: Transformed Clara's LR reactions. Had to leave during the middle of the day to go to the Student Services Office. Watched Mariola's presentation. Working on a new page.
Jenny: TriColor FACS are now up on their appropriate pages. Rest are temporarily on my notebook.
Tyler - Updated the wiki. Need to redo all of the FACS data tomorrow that Jenny did today because the red and blue colors are switched. Mariola's program works so I will use that. Tomorrow I will do transfections of a consistuitive color into wells with different confluencies that I set up today. I added Dox and Dopamine to wells today that we will FACS tomorrow.
Michelle/Divya: Miniprepped and nanodropped 12 samples. Prepped other samples for sequencing.
h3. August 8
Kenneth: planned out and did transfections for TRE eBFP2, TRE:mKate and AVPR2 validation experiments. Also threw in a co-culture of Notch/Delta cells, only with UAS:eYFP instead of UAS:citrine from Elowitz. Not expecting anything from that, but just covering all bases. Treated pDisplay-vasopressin-myc cells with anti-myc FITC Ab and prepared slide. Results suggestive. Cells were too confluent at time of fixation, many were washed off and the remaining look bad. Probably from my bootleg formaldehyde solution as well. However, remaining cells were green on the surface as well as red from delta-mcherry, while controls are not green. Seems to suggest it is being displayed...
Grant: Miniprepped the EPHB2, EFNB1, and CXCR1-TEVs-GV16 pENTR vectors assembled previously. Set up fourteen sequencing reactions of multiple Tango plasmids and a few reporter expression vectors designed to test the minCMV promoters, as well as the three ENTR vectors miniprepped. Pending affirmative sequencing results, LR reactions will begin. Because we need a number of promoters propagated and it would be nice to do LRs with the Rheoswitch system (and possibly the new Notch constructs) concurrently, I will probably wait to begin these. Cleaned the lab before the start of the work day, like a boss, or perhaps a janitor. Started propagations of a few promoters and genes that we had < 1 uL of DNA left of (and no cell stock), because if you want something done...
Tyler: Transfections
Charles: Propagated DNA we are low on. Looks like Grant also did this. So we should be stocked. And miscellanea.
Mariola: Aliquoted and Transfected UAS:EGFP and TRE:eyfp-4xFF4 experiments. Dox ladder of each. Writing 8pg REU paper and presentation.
DMC: Prepped and submitted several samples for sequencing. See Divya's Personal Notebook. Brought presents from Weiss Lab (SalI-HF, 10uL tips, 1000uL tips).
{cloak}
Week 11: August 15 - August 21
In our experimental attempt to characterize the Notch-Delta interaction, we used co-culture of CHO and Hek293 cells as well as stable cell lines of Notch-containing and Delta-containing cells from Elowitz to observe the trans-activation of Notch by Delta. Other experiments using CHO cells, which are more difficult to transfect than Hek293 using Lipofectamine, were carried out to observe the behavior of CHO transfected cells.
Week 11
h3. August 21
h4. Kenneth - Updates on Experiments:
Latest iteration of base FACS code: (incorporates Deepak's histogram update, removes the mean line on the histograms. Moves the quadrant lines to 200 for everything, since I've been seeing 200 as pretty much the max for the Blank samples. Can be easily changed.
[^auto_facsold.m] (the name is deceptive)
Results are in for dox ladder of Elowitz CMV-TO CHO cells [iGEM2011:Elowitz CHO Dox ladder], free delta induction of Elowitz receiver CHO cells. [iGEM2011:Free Delta Induction of Elowitz Receiver CHO]
FACS data is in for TRE:delta mcherry [TRE_Delta-mCherry dox ladder part 2|iGEM2011:TRE_Delta-mCherry dox ladder part 2] and TRE:mKate experiments [TRE_mKate dox ladder part 2|iGEM2011:TRE_mKate dox ladder part 2], however, due to loss of blue channel, cannot gate. Instead, using the top X% percent method to process data. The MATLAB code takes in the data and only keeps the top X% of data. In this case I used 50%. The program takes in filename, color to use as cutoff (in this case 'r'), and X for top X% (in this case 50).[^auto_facstop.m]
Co-Culture of Elowitz CHO cells: taken pictures also at 48 hours. Will bring to Weiss lab to process images. Redoing the experiment again, this time with 80:20 ratio as well. Will take pictures and FACS on Tuesday
CO-Culture of HEK and CHO cells:
\-Delta Heks and receiver CHO's: co-cultured Friday. added dox saturday. Will FACS Monday
\-Delta CHO's and receiver Hek's: same as above
\-TRE:delta Hek senders and CHO receivers: didn't have the resources to do just yet. Set up for experiment on Tuesday.
h5. Tyler
I just put up [iGEM2011:CHO Experiments]. I will try to do some analysis tomorrow, but some outside analysis would also be helpful.
h4. Charles
FACS of various things. CHO Experiment from Tyler had very unhealthy cells. Kens cocultures have yet to be analyzed.
h3. August 20
Divya - Miniprepped, Nanodropped, Inoculated. See Personal Notebook for details.
h3. August 19
Divya - Transformed, Inoculated. See Personal Notebook for details.
h3. August 18
Charles - FACS 100 samples in just under 90 minutes. Tyler's stuff had low concentrations so it ran slow, but data looks promising. Ken did Heks and Chos and both look promising. My stuff failed due to higher than intended initial cell concentration. Basically cells overgrew ran out of nutrients got sick and failed. Also made 20,000 ng of Dest 4-5.
Tyler \- The NCAD failed...so far. It's only been 24 hours after transfection, but I didn't see any EYFP or "clumping". I know that my transfection efficiency isn't zero because I also transfected some CHO cells on the same plate with Hef1A:EBFP2 and wew saw about 5-10% efficiency. I will check again tomorrow (48 hrs) using the microscope and then send them for FACS. Maybe it was a bad batch of DNA...
Other things I did today:
\-FACS for TRE:LacI and TRE:LacI-Krab (I'm generating graphs now)
\-Transfected CHO cells for Notch-Delta Co-culture
\-I was going to repeat Mariola's Mnt and CI434 activation experiments but I couldn't find the DNA, so I asked Divya to do some LR reactions
The NCAD failed...so far. It's only been 24 hours after transfection, but I didn't see any EYFP or "clumping". I know that my transfection efficiency isn't zero because I also transfected some CHO cells on the same plate with Hef1A:EBFP2 and wew saw about 5-10% efficiency. I will check again tomorrow (48 hrs) using the microscope and then send them for FACS. Maybe it was a bad batch of DNA...
Other things I did today:
\-FACS for TRE:LacI and TRE:LacI-Krab (I'm generating graphs now)
\-Transfected CHO cells for Notch-Delta Co-culture
\-I was going to repeat Mariola's Mnt and CI434 activation experiments but I couldn't find the DNA, so I asked Divya to do some LR reactions
\-Tyler
h3. August 17
Jon - Prepped Notch for full sequencing, minipreps, cell stocking, nanodropping, prepping trash for pickup.
Divya - Inoculated and LR'd NCADs, Nanodropped CPLRs. Will transform tomorrow. See personal notebook for details.
h3. August 16
Tiffany: Public wiki work forever. Don't use IE please.
Divya/Jon - In lab until 2am doing 26 minipreps, nanodrops, cell stocks, cleaning. :'(
Charles: Transfected cells with [EXP1-CH|iGEM2011:EXP1-CH]. Made graphs.
h3. August 15
Tyler: Prepared for transfections and updated the wiki. Will prepare CHO cells for NCAD phenotype experiment tomorrow. NCAD with HEK-293 cells failed (see [iGEM2011:NCAD Phenotype Verification]). Hef1A:GV16 experiments looked very good (see [Hef1A_GV16 Experiment|iGEM2011:Hef1A_GV16 Experiment]). TRE:LacI-EYFP-FF4 shows a lot of EYFP, about 25% increase if I remember correctly, but the LacI repression looks very weak. (see [TRE_LacI.Krab Experiments|iGEM2011:TRE_LacI.Krab Experiments]) We have a Hef1A:mKate that is bad, showing about 0-2.5% fluorescence. I will repeat these experiments tomorrow. The DRD2 shows very small levels of activation, but I think it is actually there (see [iGEM2011:GPCR Experiments]). Plan for tomorrow's transfections: 1.)TRE:LacI-Krab 2.)TRE:LacI 3.)miRFF4 4.)Lipo Ladder. Wednesday: CHO transfections.
More thoughts: The GV16 and LacI-Krab experiments were done on the same day. I got about 35% efficiency from the GV16 experiment. The LacI-EYFP-FF4 is showing about 20% when activated. I think the leakiness of rtTA3 is skewing our picture, so in the next round of LacI experiments, I will also include \+/\- Hef1A:rtTA3 as an added control.
Kenneth: My apologies, but I can't make the morning meeting. Made the line chart for TRE:eBFP experiment: [TRE_eBFP2 verification|iGEM2011:TRE_eBFP2 verification] looks good. Processed data from TRE:mKate experiment: [TRE_mKate verification|iGEM2011:TRE_mKate verification]. Looks weird. No increase in red, but increase in blue, almost like TRE:eBFP2. Not quite sure what went wrong here. Maybe I aliquoted the wrong DNA, maybe there's some mixup? Either way, has to be redone. Future directions: continue working on the matlab code, process FACS data for co-cultures and AVPR2 testing as well as free Delta (all done Sunday). Experiments planned:
1) Elowitz CHO cells [Initial CHO images - No Fluorescence] (so the cells don't express Delta-mCHerry without dox.) will FACS all of this Wednesday with proper scatter gating for CHO's
a. Dox induction of Delta (get a good curve showing conc of dox to amt of delta on surface
b. Addition of Free Delta to Notch-Gal4 cells and Notch+Delta cells
c. Co-culture with varying dox levels
d. If we see successful dox induction of Delta-mCherry and delta induction of Notch-Gal4/UAS, we can do the 2D ladder experiment where we vary both dox and delta levels for the Notch+Delta CHOs and see how much citrine, thus giving us a "surface"
2)redo TRE:Delta-mCherry dox ladder-GET GOOD DATA THIS TIME
3)TRE:eYFP and TRE:mKate need to be redone, I don't know if Charles wants to do this or not...
4)Immunostaining using no-coverslip technique for pdisplay-vasopressin as well as AVPR2 for troubleshooting
5)still waiting on free vasopressin for the AVPR2 tests.
6)test HRH4 with histamine
7)siRNA knockdown of N-cad.
Divya: Restriction Digested Clara's LRs. Send samples for sequencing. Labeled pRESC tubes. Got items from Weiss Lab.
Grant: Was amused at the bickering above. Designed quite a few primers\- this time around, for a Tango redesign and colored transactivators. Digested and gel extracted Sam's 4xFF4 gene entry backbone, pushing the Mnt-VP16-4xFF4 and Delta-mCherry-4xFF4 assemblies forward a bit. DNA work seems to have slowed considerably over the past few days\- we should make more (non-Tango) LRs. Ephrin stuff will hopefully be verified tomorrow. Takers?
Charles: Passed cells. Doing bunch of wiki work now.
Jon - Miniprepped and nanodropped 19 samples. Prepped SDMs for sequencing. Analyzed odd sequence results. Reinoculated Rescue 28 plasmids; put into incubator at 5:40pm, although many are already at high OD (wildly inconsistent though) so I'd check after a couple hours to make sure the E. coli don't reach stationary phase.
Week 12: August 22 - Later
Stay tuned!
Week 12
September 1
Grant: Inoculations of the aforementioned LR constructs.
August 29
Charles - CHLRs see CH notebook. H, HL, T, U and delta-ff4, H and T for ncad-2a-eyfp. inoculated propagations for HLNcad2Aeyfp, UASNcad2aeyfp, and Tre:delta.
August 28
Grant: Restarted LR reactions of 9 of the constructs listed on the Workflow (only the pEXPR_12_Hef1a-LacO_Notch-CI434-VP16 construct succeeded, possibly due to incubation time for LR reactions). Started PCRs for Tango constructs using new padding for TEV cut site.
August 25
Divya: Won.
(See Personal Notebook for details).
August 23
Charles - FACS Kens 50 50 and 80 20 Hek TRE-delta senders and CHO receivers. FACS was clean. Cells were all healthy. Lowered voltage of DAPI channel from 300 to 240 to move blank cells basal fluorescence to the bottom left corner. Laser was on, everything went according to plan. Preliminary results look promising, with increasing dox for each coculture, there was increasing FITC channel signal. Preliminary results suggest that our delta works.
August 22
Charles - FACS of Kens coculture stuff. Ambiguous results.


 "
"
