Team:Korea U Seoul/Project
From 2011.igem.org
(→Cloning of target genes) |
(→Project Details) |
||
| (41 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | {{:Team:Korea_U_Seoul/template/banner}} | ||
| + | |||
{|align="justify" | {|align="justify" | ||
| - | + | ||
|[[Image:Korea_U_Seoul_logo.png|200px|right|frame]] | |[[Image:Korea_U_Seoul_logo.png|200px|right|frame]] | ||
|- | |- | ||
| | | | ||
| - | + | ||
|[[Image:Korea_U_Seoul_team.png|right|frame|Your team picture]] | |[[Image:Korea_U_Seoul_team.png|right|frame|Your team picture]] | ||
|- | |- | ||
| Line 29: | Line 31: | ||
== '''Overall project''' == | == '''Overall project''' == | ||
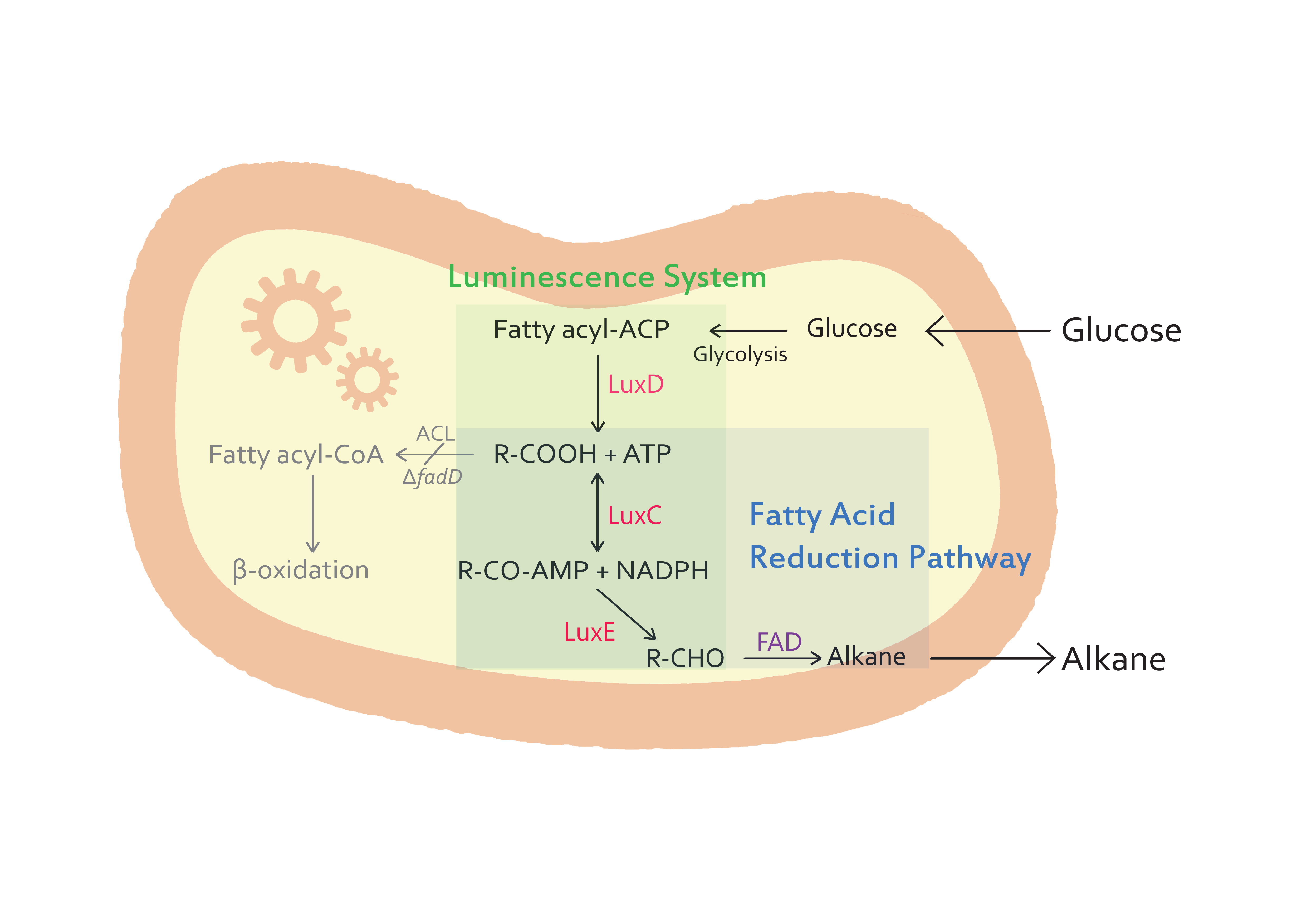
| + | <p> The goal of our project is to produce alkane chains from glucose molecules. In nature, numerous biochemical pathways and enzymes exist, making life adoptable to even extreme conditions such as volcanic regions. We focused on biochemical pathways, enzymes of glucose metabolism and luminescene luciferase from ''Vibrio harveyi'' to achieve our goal. Based on glycolysis, pyruvate oxidation, enzymes coded in luciferase genes (lux operon) and FAD from cyanobacteria, glucose is turned into alkane chain of about 14 carbon atoms in length. Synthesized fuel is functionally identical to natural petroleum and can be used as bioenergy. Produced alkane chain is part of a carbon circulation cycle as it is synthesized from glucose, in vivo. The fuel is relatively environment-friendly, unlike ordinary petroleum which increases CO2 concentration in the atmosphere. Though the production of alkanes using bioblock could be not satisfied commercially, succeeding in the synthesis of alkane chains from glucose nevertheless will show another method of producing alternative energy source. Therefore, the success of this research will contribute to global effort in reducing atmospheric CO2 levels. </p> | ||
| + | === '''Project Abstract''' === | ||
| + | '''Synthesis of Synthetic Micro-Alkanes (“Synfuels”) in Engineered ''Escherichia coli''''' | ||
| + | <br> | ||
| + | Our team concentrated on finding the solution to the world’s diminishing natural oil and gas resources and greenhouse gas emissions. The aim of our project is the production of biofuels, alkanes, using bacterial cells as factories. Alkanes, so called “Green” hydrocarbon fuels, are chemically energetically the same as petroleum-based fuels, thus no penalty for use of conventional engines is encountered from their use. For alkane biosynthesis, we designed a synthetic circuit using bacterial bioluminescence system and aldehyde decarbonylase from ''Vibrio harveyi'' and cyanobacteria, respectively. Free fatty acids in the cells firstly are reduced and converted to fatty aldehydes by ''Lux C'', ''Lux D'' and ''Lux E'' and then fatty aldehydes finally are decarbonylated and turned into alkanes. | ||
| + | == '''Project Details''' == | ||
| + | *''E.coli'' K27 strains | ||
| + | :- The purpose of our team is to synthesize alkanes from microorganisms. ''E.coli'' K27 is a suitable host for the production of alkanes because it is a FadD mutant(△''fadD''). | ||
| + | :- In our step of alkanes synthesis, the fatty acids are important intermediates. Commonly, ''E.coli'' cells contain a single acyl-CoA synthetase, which activates the conversion of free fatty acid to acyl-CoA thioester. However, ''E.coli'' K27, a FadD mutant, lacks acyl-CoA synthetase activity, which prevents substrate or product degradation by the host. So, ''E.coli'' K27 accumulates fatty acids inside the cell, and finally we can get more alkanes than other ''E.coli'' strains. | ||
| - | + | *Two-carbon compounds and fatty acids as carbon sources | |
| - | + | [[File:E.coli picture.jpg|thumb|Synthetic pathway|500px|center]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | [[File: | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | *Lux genes | |
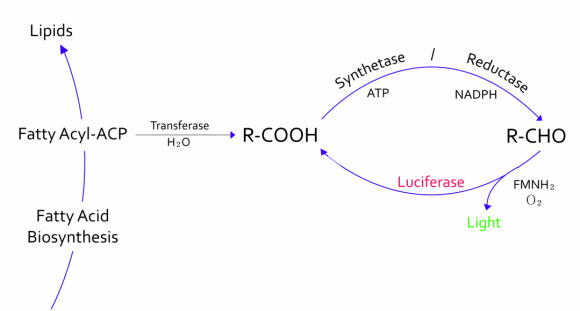
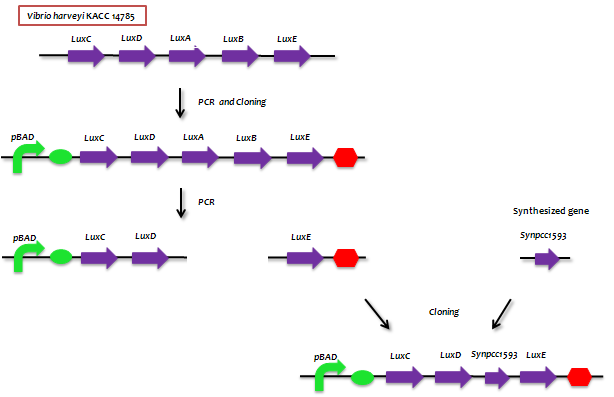
| - | + | :- The genes from bioluminescence operons have been identified, and we use some structural genes (''luxC'', ''D'', and ''E'' genes). They code for the polypeptides of the fatty acid reductase system responsible for synthesis of the fatty aldehyde substrate. | |
| + | :- There are 5 lux genes that we used. ''lux A'', ''B'', ''C'', ''D'', and ''E'' were used. ''lux A'' and ''lux B'' codes Luciferase alpha and beta subunit respectively. ''lux C'' codes Reductase, ''lux D'' codes Acyl-transferase, and ''lux E'' codes Synthetase. Luciferase alpha and beta subunit function is catalysis of the bioluminescence reaction(FMNH2 + O2 + aldehyde -> light). Reductase's function is NADPH-dependent reduction of activated fatty acyl groups to aldehyde. Acyl-transferase's function is generation of fatty acids(tetradecanoic acid) for the luminescence system. Lastly, Synthetase's function is ATP-dependent activation of fatty acids. | ||
| + | [[File:lux.jpg|thumb|Bioluminescence|500px|center]] | ||
| + | [[File:arrows.jpg|thumb|Manipulation of Lux genes|500px|center]] | ||
| + | ===Detection of hydrocarbon=== | ||
| + | *We are currently having difficulty in detecting the final product, C14. | ||
| + | :- Detection of C14 by TLC is difficult. It is not yet providing good enough results. Apparently, this is due to the fact that saturated hydrocarbons don't react with any other compounds. | ||
| + | :- By incorporating BBa_K32599 in luxCDEG, we may be able to detect hydrocarbon. | ||
| - | + | [[File:TLC method.jpg|frame less|500px|center]] | |
| + | [[File:TLC method2.jpg|frame less|500px|center]] | ||
== Results == | == Results == | ||
Latest revision as of 08:28, 5 October 2011
File:Korea U Seoul team.png Your team picture | |
| Team Example |
| Home | Team | Official Team Profile | Project | Parts Submitted to the Registry | Modeling | Notebook | Safety | Attributions |
|---|
Contents |
Overall project
The goal of our project is to produce alkane chains from glucose molecules. In nature, numerous biochemical pathways and enzymes exist, making life adoptable to even extreme conditions such as volcanic regions. We focused on biochemical pathways, enzymes of glucose metabolism and luminescene luciferase from Vibrio harveyi to achieve our goal. Based on glycolysis, pyruvate oxidation, enzymes coded in luciferase genes (lux operon) and FAD from cyanobacteria, glucose is turned into alkane chain of about 14 carbon atoms in length. Synthesized fuel is functionally identical to natural petroleum and can be used as bioenergy. Produced alkane chain is part of a carbon circulation cycle as it is synthesized from glucose, in vivo. The fuel is relatively environment-friendly, unlike ordinary petroleum which increases CO2 concentration in the atmosphere. Though the production of alkanes using bioblock could be not satisfied commercially, succeeding in the synthesis of alkane chains from glucose nevertheless will show another method of producing alternative energy source. Therefore, the success of this research will contribute to global effort in reducing atmospheric CO2 levels.
Project Abstract
Synthesis of Synthetic Micro-Alkanes (“Synfuels”) in Engineered Escherichia coli
Our team concentrated on finding the solution to the world’s diminishing natural oil and gas resources and greenhouse gas emissions. The aim of our project is the production of biofuels, alkanes, using bacterial cells as factories. Alkanes, so called “Green” hydrocarbon fuels, are chemically energetically the same as petroleum-based fuels, thus no penalty for use of conventional engines is encountered from their use. For alkane biosynthesis, we designed a synthetic circuit using bacterial bioluminescence system and aldehyde decarbonylase from Vibrio harveyi and cyanobacteria, respectively. Free fatty acids in the cells firstly are reduced and converted to fatty aldehydes by Lux C, Lux D and Lux E and then fatty aldehydes finally are decarbonylated and turned into alkanes.
Project Details
- E.coli K27 strains
- - The purpose of our team is to synthesize alkanes from microorganisms. E.coli K27 is a suitable host for the production of alkanes because it is a FadD mutant(△fadD).
- - In our step of alkanes synthesis, the fatty acids are important intermediates. Commonly, E.coli cells contain a single acyl-CoA synthetase, which activates the conversion of free fatty acid to acyl-CoA thioester. However, E.coli K27, a FadD mutant, lacks acyl-CoA synthetase activity, which prevents substrate or product degradation by the host. So, E.coli K27 accumulates fatty acids inside the cell, and finally we can get more alkanes than other E.coli strains.
- Two-carbon compounds and fatty acids as carbon sources
- Lux genes
- - The genes from bioluminescence operons have been identified, and we use some structural genes (luxC, D, and E genes). They code for the polypeptides of the fatty acid reductase system responsible for synthesis of the fatty aldehyde substrate.
- - There are 5 lux genes that we used. lux A, B, C, D, and E were used. lux A and lux B codes Luciferase alpha and beta subunit respectively. lux C codes Reductase, lux D codes Acyl-transferase, and lux E codes Synthetase. Luciferase alpha and beta subunit function is catalysis of the bioluminescence reaction(FMNH2 + O2 + aldehyde -> light). Reductase's function is NADPH-dependent reduction of activated fatty acyl groups to aldehyde. Acyl-transferase's function is generation of fatty acids(tetradecanoic acid) for the luminescence system. Lastly, Synthetase's function is ATP-dependent activation of fatty acids.
Detection of hydrocarbon
- We are currently having difficulty in detecting the final product, C14.
- - Detection of C14 by TLC is difficult. It is not yet providing good enough results. Apparently, this is due to the fact that saturated hydrocarbons don't react with any other compounds.
- - By incorporating BBa_K32599 in luxCDEG, we may be able to detect hydrocarbon.
 "
"