Team:Calgary/Project/Promoter/Bioinformatics
From 2011.igem.org
| Line 86: | Line 86: | ||
<p><a href="http://mummer.sourceforge.net/">MUMmer</a> is an open source program which can align genomes within a matter of seconds. We used MUMmer to verify our assumption that there was a substantial degree of homology between <i>Pseudomonas putida</i> and <i>fluorescens</i>. This assumption is important, because its corollary is that there is a limited number of genes unique to either species. The fewer unique genes there are, the easier it is to find the right one.</p> | <p><a href="http://mummer.sourceforge.net/">MUMmer</a> is an open source program which can align genomes within a matter of seconds. We used MUMmer to verify our assumption that there was a substantial degree of homology between <i>Pseudomonas putida</i> and <i>fluorescens</i>. This assumption is important, because its corollary is that there is a limited number of genes unique to either species. The fewer unique genes there are, the easier it is to find the right one.</p> | ||
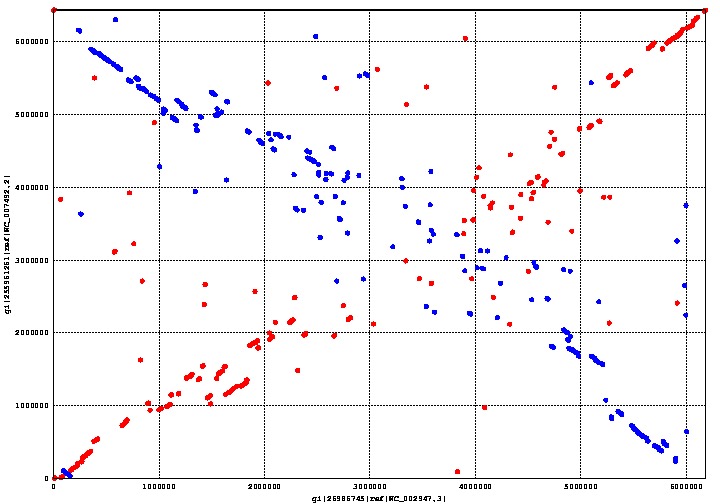
| - | </html>[[image:Calgary2011_MummerPlot.jpg | + | </html>[[image:Calgary2011_MummerPlot.jpg|thumb|600px|center|<b>Figure 6</b><p>Figure 6 shows the alignment between <i>Pseudomonas putida</i> (x-axis) and <i>fluorescens</i> (y-axis). Linear regions are more conserved and scattered regions are less conserved.</p>]]<html> |
<p>The graph above shows the genome alignment between <i>Pseudomonas putida</i> and <i>fluorescens</i>, where the earlier is on the x-axis and the latter on the y-axis. Regions conserved between both species form a straight line going from the bottom left corner to the top right corner; the line going from the top left corner to the bottom right corner is also a conserved region between both species, except that it is backwards. Areas with a more scattered distribution indicate a more random, and therefore less homologous, aligned region between the two species.</p> | <p>The graph above shows the genome alignment between <i>Pseudomonas putida</i> and <i>fluorescens</i>, where the earlier is on the x-axis and the latter on the y-axis. Regions conserved between both species form a straight line going from the bottom left corner to the top right corner; the line going from the top left corner to the bottom right corner is also a conserved region between both species, except that it is backwards. Areas with a more scattered distribution indicate a more random, and therefore less homologous, aligned region between the two species.</p> | ||
Revision as of 07:40, 28 September 2011









NA-Sensitive Promoters using Bioinformatics and qRT-PCR

Introduction
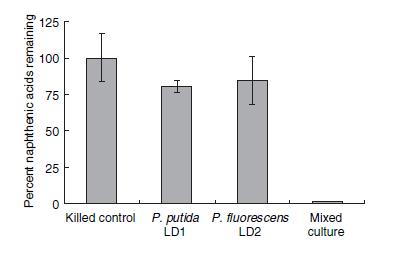
A recent survey (footnote: Del Rio) found that two bacterial strains LD1 and LD2 (later identified as Pseudomonas fluorescens and putida) were capable of almost completely degrading a mixture of naphthenic acids when co-cultured together.
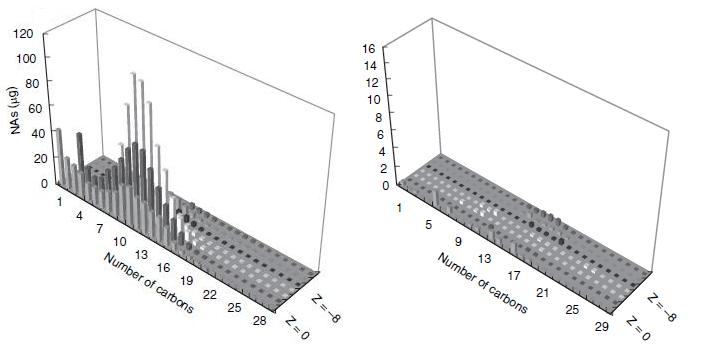
Figure 2.
Figure 2 shows how the degradation affected the concentrations of specific naphthenic acids of varying carbon number and saturation, as measured by GC-MS. The chart on the left of figure 2 shows the initial concentrations of each of the naphthenic acids, and the chart on the right shows the concentration of the naphthenic acids after four weeks of a LD1-LD2 co-culture. Based on the decreased height and scale, the figure demonstrates that naphthenic acids were more or less indiscriminately degraded by the co-culture.
When both figure 1 and 2 are taken together, the most likely conclusion is that the genes responsible for the degradation are spread across the two species. Furthermore, there exists a collection of essential genes in this degradation pathway which are unique to one species and not present in the other. The degradation pathway has not been experimentally confirmed, but it is believed to be some sort of metabolic pathway which involves beta-oxidation (footnote: Del Rio). In any case, the promoters upstream of these essential genes would be a likely place to find a naphthenic acid promoter - and indeed, our later experiments found that they were. A weakness of the bioinformatics search was that LD1 and LD2 have not yet been sequenced, and therefore we assume similarity between them and corresponding strains that have been sequenced; this weakness complicated primer design but was not prohibitive to the project.
Abstract
Three online algorithms (DarkHorse, ProteinWorld DB, and Pseudomonas.com) were used to find target gene candidates in silico, and a fourth (MUMmer ) was used to confirm experimental hypotheses. Using the data from these sources, we were able to construct a short list of gene candidates which were then processed through a quantative Real-Time Polymerase Chain Reaction (qRT-PCR) to verify gene expression as a response to naphthenic acids. The qRT-PCR found that Enoyl CoA Hydratase, from Pseudomonas putida, was expressed as a response to naphthenic acids.
The characterization of its promoter can be found below.
DarkHorse and ProteinWorld DB Results
DarkHorse is an online database for finding phylogenetically atypical proteins in various microbial species. Generally, phylogenetic atypicality can be useful for predicting the horizontal transfer of genes; in this bioinformatics survey, this also functions to provide a list of relatively unique genes in each of the strains in Pseudomonas putida and fluorescens.
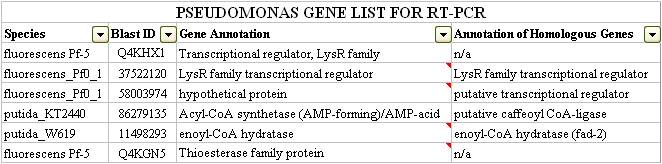
Figure 3 shows a sample of the input used for our DarkHorse Query. The list returned by querying the database for genes in LD1 and LD2 was 258 records long. Each record contained the GI number, strain, and gene annotation for the query species and its best phylogenetic match. To sort through the data, we started by collecting data about the best match species and the gene annotations. Using the data about the species, we found that some of them had known degradation pathways and were naturally occurring in tailings. We were especially interested in such species when they donated a predicted transcription factor or metabolites related to fatty acid degradation. Fatty acids are structurally similar to naphthenic acids and therefore may have similar promoters. We also chose to short-listed enoyl CoA hydratase because a paper we read recently suggested that hydratases are known to degrade similar compounds such as caffeic acid and cinnanoic acid.
Later on, we also found Protein World DB, a database for finding unique genes across a set of compared species. This database did not provide information on horizontal transfer, but we looked for the same kind of genes as before.

Picture showing the layout of the ProteinWorld database website. Source: <a href="http://darkhorse.ucsd.edu/search.shtml">http://darkhorse.ucsd.edu/search.shtml/</a>.
At the end of looking through these two programs, we were ready to try to verify their transcription via qRT-PCR. The genes we tested are listed below:
Quantative RT-PCR Results
Primers for the Polymerase Chain Reaction (PCR) were designed with the following guidelines in mind:
- Melting Temperature: 70-74°C (optimally 72°C)
- Primer Length: 22-24 (optimally 24)
- Product Length: 150-250 base pairs (optimally 200)
- No runs of 4 or more of the same nucleotide letter in either the forward or reverse primer.
- The primers have a GC clamp at the end (ie. end with G, C, GC, or CG)
- The last 5 nucleotides contain 3 or 4 GC's.
- GC Content between 40-60% (optimally 50%)
- All unintentional matches calculated using Primer Blast are at least 25% mismatched.
Cell cultures of LD1 and LD2 were grown in minimal media in mixtures containing either 80mg/L of naphthenic acids or Casamino acids. We used several kits from Qiagen, including RNA Protect and RNEasy, etc. 16s rRNA, a constitutively expressed conserved gene found in both LD1 and LD2, was used as a control for the experiment.
Appendix A: Pseudomonas.com Results
The genes found from a subsequent bioinformatic search on Pseudomonas.com were not used in the RT-PCR, but have been included as an appendix for the iGEM community. Pseudomonas.com is a database specializing in the annotation and collection of information related to the Pseudomonas genus, but it also has a comparative genome search tool which claims to allow the location of genes unique to one species and not the other. The list, which has not been completely processed yet, can be found in this .zip file in both .xls and .xlsx format (the latter is recommended). One of the most interesting genes found on the list is annotated as glyoxalase/bleomycin resistance protein/dioxygenase, but is similar to a ring-cleaving dioxygenase also found in putida.
Appendix B: MUMmer Findings
MUMmer is an open source program which can align genomes within a matter of seconds. We used MUMmer to verify our assumption that there was a substantial degree of homology between Pseudomonas putida and fluorescens. This assumption is important, because its corollary is that there is a limited number of genes unique to either species. The fewer unique genes there are, the easier it is to find the right one.
The graph above shows the genome alignment between Pseudomonas putida and fluorescens, where the earlier is on the x-axis and the latter on the y-axis. Regions conserved between both species form a straight line going from the bottom left corner to the top right corner; the line going from the top left corner to the bottom right corner is also a conserved region between both species, except that it is backwards. Areas with a more scattered distribution indicate a more random, and therefore less homologous, aligned region between the two species.

 "
"