Team:Calgary/Project/Project/Chassis/Microalgae
From 2011.igem.org
Emily Hicks (Talk | contribs) |
Emily Hicks (Talk | contribs) |
||
| Line 18: | Line 18: | ||
Looking through the registry, we also realized that for future team to make use of microalgae, there were several parts that would be required. The regitsry contains a few appropriate selectable markers (kanamycin and gentamycin), a strong constitutive 35S CaMV promoter (BBa_K509000), as well as a visual GUS reporter (BBa_K330002). What was needed we felt was an inducible promoter and a more quantitative reporter gene. To this end, we biobricked the Hsp70A/RbcS2 promoter from Chlamtdomonas reinhardtii (BBa_K640001), which is inducible by light/ heat shock. We also biobricked a gene for a codon optimized algal luciferase. We submitted these parts to the registry, and did some preliminary characterization data. | Looking through the registry, we also realized that for future team to make use of microalgae, there were several parts that would be required. The regitsry contains a few appropriate selectable markers (kanamycin and gentamycin), a strong constitutive 35S CaMV promoter (BBa_K509000), as well as a visual GUS reporter (BBa_K330002). What was needed we felt was an inducible promoter and a more quantitative reporter gene. To this end, we biobricked the Hsp70A/RbcS2 promoter from Chlamtdomonas reinhardtii (BBa_K640001), which is inducible by light/ heat shock. We also biobricked a gene for a codon optimized algal luciferase. We submitted these parts to the registry, and did some preliminary characterization data. | ||
| - | </html>[[File:UCalgary2011_Promoter.png]]<html><br> | + | </html>[[File:UCalgary2011_Promoter.png]][[File:UCalgary2011 Algae Luciferase.png]]<html><br><br> |
Revision as of 08:31, 28 September 2011









A Chassis Using Native Tailings Pond Species

Microalgae Tools
In terms of microalgae, the first things we needed were protocols for various procedures. We wanted to eventually use our microagae strain to construct a promoter library out of nuclear and chloroplast DNA. As such, transformation as well as nuclear DNA and chloroplast extraction protocols were necessary. Unfortunately, our strain of microalgae, Dunaliella tertiolecta, has not been transformed very often in the literature, and as such we had to spend some time optimizing literature protocols. All microalgae protocols we used can be found in our protocol section.
Transformation
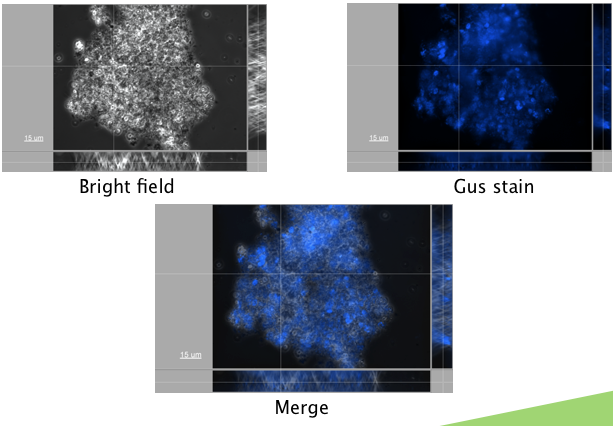
An appropriate reporter also needed to be found. A reporter construct containing GFP downstream of a Nopaline Synthase (NOS) promoter was first transformed. Autofluorescence levels were found to be too high, however, to detect fluorescence above the background, making this a poor choice for a reporter. A GUS reporter commonly used in plants, and available in the registry (BBa_K330002) was tried next. GUS (beta-glucuronidase) is an enzyme from E. coli, that is frequently used a s a plant reporter. A construct conatining the GUS reporter gene, downstream of the NOS reporter was successfully transformed (figure x.)
New Microalgae Parts
Looking through the registry, we also realized that for future team to make use of microalgae, there were several parts that would be required. The regitsry contains a few appropriate selectable markers (kanamycin and gentamycin), a strong constitutive 35S CaMV promoter (BBa_K509000), as well as a visual GUS reporter (BBa_K330002). What was needed we felt was an inducible promoter and a more quantitative reporter gene. To this end, we biobricked the Hsp70A/RbcS2 promoter from Chlamtdomonas reinhardtii (BBa_K640001), which is inducible by light/ heat shock. We also biobricked a gene for a codon optimized algal luciferase. We submitted these parts to the registry, and did some preliminary characterization data.
Characterization of the Hsp70A/RbcS2 promoter (BBa_K640001)

 "
"