Team:Calgary/Outreach/Collaboration
From 2011.igem.org









Collaboration

Another important part of iGEM is helping other teams through collaboration. As in previous years, we had the opportunity to meet up with the other Alberta teams (Lethbridge and Alberta) at several points during the Summer. We had the opportunity to ask questions, do practice presentations for each other, as well as give each other ideas for each specific project. This was an awesome resource, especially given that all three teams are looking at oil sands and/or energy-related projects. September 24-25th, all Alberta iGEM teams met for the 3rd annual aGEM competition, where we all did a mock iGEM presentation and got questions and feedback from the other teams as well as from a group of judges. This event was organized by AITF, one of our major sponsors.

In discussions with the Lethbridge iGEM team, we discovered that we're both interested in the viability of E. coli in tailings pond samples. The Lethbridge team wants to eventually house their system in E. coli; although we do not, we are still interested in it in case we encounter difficulties with pseudomonas. We have done previous viability assays, however only in commercial NA mixtures. Tailings ponds have a large amount of different toxic compounds in them other than NA's. In addiion, different tailings pond samples can have a very different composition, depending on which pond they were taken from, and even which section or depth of the pond they were taken from. Because of this, we decided that it may be interesting to test the viability of E. coli in different samples of tailings pond water. The Lethbridge team has access to one sample of tailings pond water, while we have access to two others from different locations. We decided to access E. coli viability in all three tailings ponds to see if we can observe any differences.
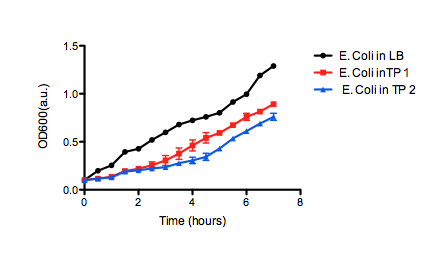
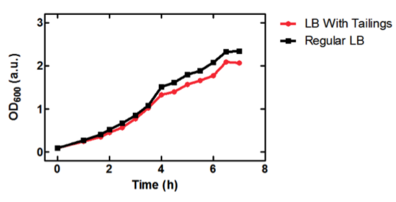
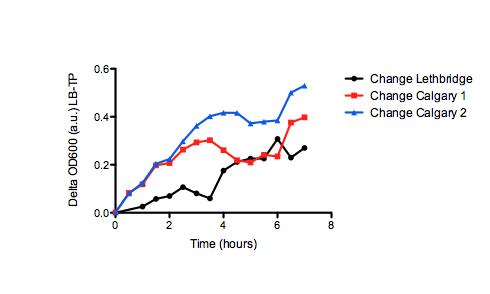
On the right, we see the data set obtained from our team, where we graphed OD over time for our E. coli grown in two different samples of oil sand tailings ponds, as compared to just LB as a control. Points are the average of two data points and standard error bars are indicated where necessary. The graph on the left shows the University of Lethbridge data set, which again shows OD over time. The graph on the bottom shows the change in OD for the cells grown in the tailings pond samples, as opposed to the LB only control.

In discussions with the Lethbridge iGEM team, we discovered that we're both interested in the viability of E. coli in tailings pond samples. The Lethbridge team wants to eventually house their system in E. coli; although we do not, we are still interested in it in case we encounter difficulties with pseudomonas. We have done previous viability assays, however only in commercial NA mixtures. Tailings ponds have a large amount of different toxic compounds in them other than NA's. In addiion, different tailings pond samples can have a very different composition, depending on which pond they were taken from, and even which section or depth of the pond they were taken from. Because of this, we decided that it may be interesting to test the viability of E. coli in different samples of tailings pond water. The Lethbridge team has access to one sample of tailings pond water, while we have access to two others from different locations. We decided to access E. coli viability in all three tailings ponds to see if we can observe any differences.
Protocol
50 mL of LB medium was made with tailings water and filtered through MILLEX filter Unit GS MF-Millipore MCE Membrane 0.22 µm in order to sterilize it and appropriate antibiotics were added. The tailings LB was inoculated with E. coli DH5α cells containing BBa_K331009 to an OD600 of 0.1. The flask was incubated at 37˚C with shaking for 7 hours, during which photometric readings were taken every 30. Photometric readings were taken using the Pharmacia Biotech Ultrospec 3000, as the OD600 readings reached 1.0 the solutions was diluted with LB media to keep the OD600 reading between 0.1 and 1.0. The same protocol was used to observe cell growth of E. coli DH5α cells containing BBa_K331009 in 50 mL of LB medium made with MilliQ H2O.Results


On the right, we see the data set obtained from our team, where we graphed OD over time for our E. coli grown in two different samples of oil sand tailings ponds, as compared to just LB as a control. Points are the average of two data points and standard error bars are indicated where necessary. The graph on the left shows the University of Lethbridge data set, which again shows OD over time. The graph on the bottom shows the change in OD for the cells grown in the tailings pond samples, as opposed to the LB only control.
Discussion
Because we saw so much variability in growth from the two data sets, we decided to graph the change in tailings ponds viability as compared to the control (just grown in LB) for both sets of data (the bottom graph). This was interesting, as it shows that Lethbridge saw a much smaller difference in viability between E. coli cultures grown in tailings pond samples v.s. LB. This suggests that some tailings ponds may be more suitable for E. coli growth than others.
 "
"