4
From 2011.igem.org

Nina Jiayue Zhu
- Synthetic Graphite normally has a high electrical conductivity than the natural ones.
- the process involves turning amorphous carbon into crystal under extremely high temperature.
- no evidence shows that carbon(element) can be either iuput or output of bacterial metabolism.
- therefore, we can pass this topic
- anti-venom
- traditional way of anti-venom production
1. injecting venom or detoxified venom into a horse (tiny amount, multiple times)
2. after the antigen growing period, contract the horse blood plasma
3. use stomach digestive enzyme to breakdown the anti-venom protein into smaller globin molecules
4. use (NH4)2SO4 to salt out the globin (purification) [1]
- oxides like K2MnO4 can neutralise venom by denaturing the polypeptide chains
- complex ligase like AuCl2 can denature venom by binding with them, when preventing venom entering the tissues [2]
- immune system has a stronger response to venom [3]
when the mast cells are stimulated, they release histamine
histamine can subside the venom
- histamine producing bacteria:
found in tuna
18 types of bacteria such as Clostridium perfringens,etc
anaerobic bacteria
- to conclude
1. decide the working mechanism (toxicology) of a specific type of venom
attack neurons? brain cells? cardiovascular system? respiratory system?
2. does it react with histamine?
3. bacterial production of histamine
REFERENCES:-
[1] <a href="http://en.wikipedia.org/wiki/Antivenom">http://en.wikipedia.org/wiki/Antivenom</a>
[2] <a href="http://life.91sqs.com/html/zazhi/yixueyushehui/2011/0113/1453.html">http://life.91sqs.com/html/zazhi/yixueyushehui/2011/0113/1453.html</a>
[3] “ Development of antibody against Naja naja atra venom using phage display and single-chain Fv antibody technology ”
Master Graduation Paper NUK
<a href="http://ethesys.nuk.edu.tw/ETD-db/ETD-search-c/view_etd?URN=etd-0825110-170246">http://ethesys.nuk.edu.tw/ETD-db/ETD-search-c/view_etd?URN=etd-0825110-170246</a>
Frank Machin
- First, I began to look into the possible production of the alkaloid isorhy, as was brought to my attention by Nina and Si
- It is rumoured to be a potential treatment for Parkinson's and it would make a good project if this were to be produced by bacteria
- On further research it turns out that the evidence for this drug as a treatment is weak and there is no information available about the gene or genes that encode it, so the idea was dropped
- After being inspired by a student from the Royal College of Arts who presented us with her work on a project to create a living dress, I began to research the notion of a melanin tattoo, so that alpha-melanin stimulating hormone is applied to the skin and held in place until the skin darkens in the shape of the template. The alpha-MSH could be produced by bacteria.
- the alpha-MSH gene is produced as one gene that also contains beta-MSH and gamma-MSH that are made available through post-transcriptional processing, so only the alpha-MSH region is required as it is the best characterised and has been expressed before
- Once the alpha-MSH is expressed, it can be collected, soaked into silk (for example) that is cut into a pattern and will allow the hormone to diffuse into the skin, producing a (probably temporary) tattoo.
From: http://en.wikipedia.org/wiki/Melanocyte-stimulating_hormone
- However, it seems that alpha-MSH is a rather powerful aphrodisiac and so a different hormone will have to be chosen, in addition, it seems that the hormone is unlikely to penetrate the skin as there are many different layers as well as proteases secreted by the skin - alpha-MSH, or its analogues are already used as tanning solutions and the analogues are considerably stronger - so then, bacteria could express the gene for melanotan - which is a cyclic lactyam analogue, and this will be very difficult to express, as a method will have to be found for cyclisation. - One further problem:
"As of 2010 no compound incorporating the melanotan II peptide has ever been approved for use by any governmental drug regulatory bodies outside of clinical trials. Unlicensed and untested powders sold as "melanotan II" are found on the Internet and are reported to be used by thousands of members of the general public. Multiple regulatory bodies have warned consumers the peptides may be unsafe and ineffective in usage with one regulatory agency warning that consumers who purchase any product labeled "melanotan" risk buying a counterfeit drug. Medical researchers and Clinuvel Pharmaceuticals, the company developing the related peptide afamelanotide, has warned consumers that counterfeit products sold using the names "melanotan I and II", "pose a hazard to public health"." From http://en.wikipedia.org/wiki/Melanotan
- alpha-MSH will have to be used, but this time with the novel method for crossing the skin: Transdermal iontophoresis. This is a non-invasive way for hydrophilic proteins to be transported across the skin but I do not know what kind of resolution is possible with this device. Whatever the pattern achieved, be it a nice dot or a blotted smudge, the students from the RCA will surely help to make it look pretty.
Christopher Schoene
We had a briefing today chaired by James. Key action points of the day were to organize our CID's, organize who was in charge of each team aspect and discuss the problems that we thought could be solved by Synthetic Biology. Once these problems had been discussed, we each chose one project that another team member came up with to research. We also were introduced to Professor Freemont and Professor Kitney who gave us an insightful talk about what awaits us. A trip to the Royal Society of Science exhibition ended up turning into a lunch in China town (the exhibition actually starts tomorrow) and we talked to Nicola Morgan who is interested in investigating the use of bacteria in making patterns on clothing.
Vampiric bacteria:
-The aspect of a Vampiric bacteria that is designed to get
rid of blood clots produced by trauma induced clotting or during complex
medical procedures is intriguing.
-Expression of Hirudin is possible in systems
such as E. coli. In 2007 Berkeley produced a chassis for a E. coli that could
be introduced into the blood stream after inactivation.
-However, it is
difficult to have the non-viable cell lysis occur in the correct location and
therefore an anticoagulant could just as well be injected into the patient.
-For
this to work, we would require an expression system that is able to express
Hirudin (produced usually by leech salivary glands and has been successfully
expressed in E. coli [1]), express anti-angiotensin (it is possible to express Fab fragments in E. coli [2]) and targeting the fibrin (can be done by expressing Tissue plasminogen activator).
-The idea would be to have the chassis recognize a blood clot or an area of damage and prevent clotting and/or clear clots. A method for having the system recognize when to secrete hirudin would be by having the bacteria sense trauma related chemokines and have the chassis secrete the protein only when it senses above a certain threshold of these chemokines or we could try to express protease-activated receptors (GPCR) that are cleaved by activated thrombin (the target of hirudin). Direct application would only benefit over the use of leeches in that the chassis is more aseptic then a leech bite.
The biggest issue remains the fact that for this to work we would have to inject the patient with living E. coli that can evade the human immune system.
A new method of boosting biosynthesis has been obtained through the use of RNA scaffolds:
http://www.sciencemag.org/content/early/2011/06/22/science.1206938
Reference:
[1]Shuhua Tan et al., “Efficient expression and secretion of recombinant hirudin III in E. coli using the L-asparaginase II signal sequence,” Protein Expression and Purification 25, no. 3 (August 2002): 430-436.
[2]Saad A Masri et al., “Cloning and expression in E. coli of a functional Fab fragment obtained from single human lymphocyte against anthrax toxin,” Molecular Immunology 44, no. 8 (March 2007): 2101-2106.
[3]Ji Qiu, James R. Swartz, and George Georgiou, “Expression of Active Human Tissue-Type Plasminogen Activator in Escherichia coli,” Applied and Environmental Microbiology 64, no. 12 (December 1998): 4891-4896.
Si Chen
Problem: Convert fallen leaves into useful products
· Aquatic hyphomycetes has been recognized as critical for controlling the process of leaf litter breakdown.
· The activity of this fungi is affected by
1. C:N ratio
2. Lignin content
3. pH of water, temperature, abundance of nutrient (i.e. O2)
· They produce B-glucosidase (bgaf2), Cellobiohyhrolase (cbhI family), B-xylosidase (xlnR)and phenoloxidase (Pox2) to promote leaves degradation.
· As leaves decay, they produce heat. And leaves will decompose into an excellent organic soil amendment that can be used as a soil conditioner.
· The decomposition process is slow (i.e. leaves require 5 months to 2 years to decompose), could combine with Nick’s gene expression amplification?
However, rapid decomposition would consume large amount of O2 and create anaerobic condition. Could we engineer all these into a anarobic bacteria like Facultative anerobes ?
· Reference:
[1] FEMSMicrobiolLett 264(2006)246–254, DOI:10.1111/j.1574-6968.2006.00462.x
[2] E.N. Tamayo et al. / Fungal Genetics and Biology 45 (2008) 984–993, doi:10.1016/j.fgb.2008.03.002
[3] APPLIED AND ENVIRONMENTAL MICROBIOLOGY, June 2008, p. 3481–3489, doi:10.1128/AEM.02893-07
[4] Mutagenesis Advance Access published June 15, 2006, doi:10.1093/mutage/gel025
[5] http://herbarium.usu.edu/fungi/funfacts/decay.htm
[6] http://onlinelibrary.wiley.com/doi/10.1002/iroh.201111355/pdf
Yuanwei Li
Fuel from food waste
Microbes in food waste like heterotrophs, cyanobacteria, microalgae and purple bacteria produce biohydrogen. Hydrogen has more potential energy than petrol. Hence, food waste can be turned into valuable energy. Fermentative bacteria use carbohydrates like sugar to produce hydrogen and acids. Purple bacteria, use light to produce energy (photosynthesis) and make hydrogen to help them break down molecules such as acids. http://www.sciencedaily.com/releases/2008/07/080716204805.htm
Hydrogen is produced by feeding waste products from a chocolate factory to Escherichia coli bacteria. E Coli ferment the sugars in the chocolate waste, which generated organic acids so toxic to the bacteria that they began converting formic acid to hydrogen. http://environment.about.com/od/renewableenergy/a/chocolatefuel.htm
Cellulose waste can be converted to energy by using enzyme cellulase. The gene that codes for cellulase has been isolated and grown in large quantities by E. coli. A number of photosynthetic bacteria, nonphotosynthetic bacteria, cyanobacteria, and green, red, and brown algae produced the enzyme hydrogenase, which is necessary to make hydrogen.
http://www.accessexcellence.org/RC/AB/BA/Future_Fuel.php
Feather-Eating Bacteria
Bacillus licheniformis Strain PWD-1 breaks down feather into a feather-lysate compound. Feather-lysate provides a low-cost, highly digestible protein source for livestock feed. Bacillus has also been shown to secrete a keratinase enzyme that hydrolyzes proteins such as collagen, elastin, and keratin. Potential application in breakdown of livestock carcasses. The gene encoding the enzyme keratinase of Bacillus licheniformis is kerA. http://www.accessexcellence.org/RC/AB/BA/The_Smell_of_Wealth.php
http://aem.asm.org/cgi/content/abstract/61/4/1469
Nicolas Kral
problem: How to make C3 plant operating in sunny and arid areas or how to reduce photorespiration
solution: Create a bacteria which penetrates plant cells, creates high concentration of HCO3− and packages it into vesicles, inactive Carbonic anhydrase is added to the vesicles, releases vesicles with chloroplast localisation signal, releases vesicles into the chloroplast, upon fusion CA is activated and changes HCO3− into carbon dioxide, which is then highly concentrated in a chloroplast and reduces rate of O2 binding to the Rubisco simply by increasing concentration of CO2.
Chassis: E.coli or Sinorhizobium meliloti
Bacterial infection: Nod factors
Bacteria of Rhizobium spp. are capable of infecting a plant and forcing it to develop an extra organ - Nodule, where these bacteria then intracellularly (a bit like organelles) reside. They do this to develop a mutualistic relationship with plant. We could use this mechanism of infection and acceptance by using the entire "Nod box" a cluster of genes involved in signalling to the plant to allow entry through the specially deformed root (induced by the Nod factors) or by crack entry. Each of the two mechanisms involves plant release of the flavonoids in the first place to trigger the Nod factors in the first place.
File:Rhizobium.gif
problems:
-A lot of plants do not have Nod factor receptors, as wild type Rhizobium infects only legumes, so we would have been restricted to legumes as well. Also there is specificity among different Nod factors and their receptors on the plants meaning that not every Nod box containing bacteria could infect every plant.
-In theory inserting a whole "Nod box" of genes into E. coli should enable E.coli to function in relation to the plant much in the same way as Rhizobium does, however we can not be sure of that, even though there is evidence that some genes in Rhizobium (NodD) have orthologues in E. coli (glmS).
-Plant accepts Rhizobium as a symbiont and expects to get something from it, therefore if we were to use Rhizobium as chassis we could leave the initial nitrogenase function intact, however there might be a problem using E. coli as it would not be capable of fixing nitrogen the plant might not accept its infection thread.
-Rhizobium forces plant to form nodule on the root, however ideally we would want to set up infection into the leaves. Maybe possibility to send vesicles through the xylem to the leaves, however vesicles would face problem of crossing plant cell wall.
Accumulation of HCO3− and packaging into the vesicles: CaA and carboxysome
A lot of cyanobacteria / algae, use specialised carboxysomes to accumulate HCO3− through a number of HCO3− transporters and carbon dioxide converting enzyme Carbonic anhydrase which performs interconversion of CO2 and HCO3−. Different genes in C. reinhardtii (cupA, cupB) act as transporters of CO2 and automatically convert it to HCO3−. There is a number of other transporters utilised by cyanobacteria, but these just transport HCO3−
and do not convert it to CO2, and therefore are not useful to us. Then a number of genes involved in carboxysome production would have to be included in the chassis as well. Also normal carboxysome in a cyanobacterium contains a number of other protein products to convert CO2, however these are not necessary as carbon fixation would be performed by the plant itself. Finally a CaA - carbonic anhydrase converting HCO3− to CO2 would be included, also Cso3 a Carbonic anhydrase embedded in the carboxysome membrane would be present.
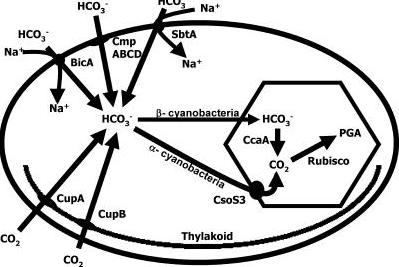
However it needs to be inactive within the carboxysome/vesicle and active only upon entry into chloroplast. Therefore possible fusion protein with 3 domains could be created containing CA on the inner end, then transmembrane subunit and a transit/fusion peptide targeting it to the chloroplast. Upon fusion into chloroplast the fusion protein would be cleaved and CA would become active.
- Creation of carboxysome ( a whole "organelle") within a chassis not previously having any.
- Creating vesicles out of carboxysome, which would not release any of its content out into bacterial cytoplasm (whole compartmentalisation would not work)
- This also raises a question of what concentration of HCO3− can be transported within one vesicle, if the concentration is too low it will not function.
Could be largely based on the OMV-outer membrane transport, which has been worked out by igem team paris 2009. However a number of outer-transit/fusion peptides would have to be different to ensure targeting towards chloroplast and succesful fusion into the chloroplast.
problems:
- usual transit peptide used for fusion protein targeting from cytoplasm into chloroplast (5kDa Rubisco subunit) might not work in targeting of the wholve vesicle into the chloroplast.
- previous igem team have developed OMV to transport proteins from cytoplasm to another bacteria. In this situation however we would use OMV to transport concentrated solution from carboxysome - "organelle", therefore the OMV itself might not work on our setup.
References: Moroney, J.V. & Somanchi, A., (1999). How Do Algae Concentrate CO2 to Increase the Efficiency of Photosynthetic Carbon Fixation? Plant Physiology, 119 (1), 9 -16.
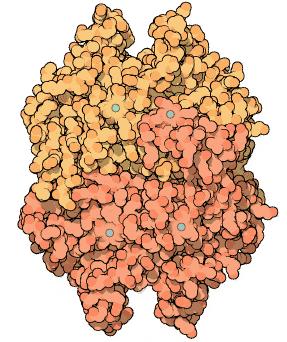
Goodsell a S. Dutta, “Carbonic Anhydrase”, RCSB Protein Data Bank (january, 2004), http://www.pdb.org/pdb/101/motm.do?momID=49.
Nod factor interaction picture taken from: http://www.glycoforum.gr.jp/science/word/saccharide/SA-A02E.html
 "
"