Team:Bielefeld-Germany/Protocols
From 2011.igem.org
Transformation via electroporation
- Thaw 50 µL competent E.coli cells on ice, dilute with icecold 50 µL glycerol (10 %) if necessary
- Add 0.5-5 µL plasmid to 50 µl electrocompetent cells
- Store cells on ice for 1 minute
- Electroporate at U = 2.5 kV, C = 25 µF, R = 200 Ώ
- Transfer transformation reaction to 450 µL SOC-Medium and shake 1 h at 37 °C
- Centrifuge 2 min at 800 rpm and plate on selective LB-Medium
Transformation of Single Step (KRX) Competent Cells by Promega
using [http://www.promega.com/~/media/Files/Resources/Protocols/Technical%20Bulletins/101/Single%20Step%20Competent%20Cells%20Protocol.ashx protocol for E. coli KRX single step competent cells by Promega]
- Remove Single Step (KRX) Competent Cells from –70 °C, and place on ice for 5 minutes or until just thawed.
- Add 1–50 ng of DNA (in a volume not greater than 5 μL) to the Single Step (KRX) Competent Cells. Move the pipette tip through the cells while dispensing. Quickly flick the tube several times. Do not vortex!
- Immediately return the tubes to ice for 5–30 minutes
- Heat-shock cells for 15–20 seconds in a water bath at exactly 42 °C. Do not shake.
- Immediately place the tubes on ice for 2 minutes.
- Add 450 μL of room-temperature SOC medium to each transformation reaction, and incubate for 60 minutes at 37 °C with shaking (approximately 225 rpm). For best transformation efficiency, lay the tubes on their sides and tape them to the platform.
- For each transformation reaction, we recommend plating 100 μL of undiluted cells and 1:10 and 1:100 cell dilutions on antibiotic plates. Incubate the plates at 37 °C overnight.
Standard BioBrick Assembly
modified from [http://openwetware.org/wiki/Silver:_BB_Strategy Silver lab]:
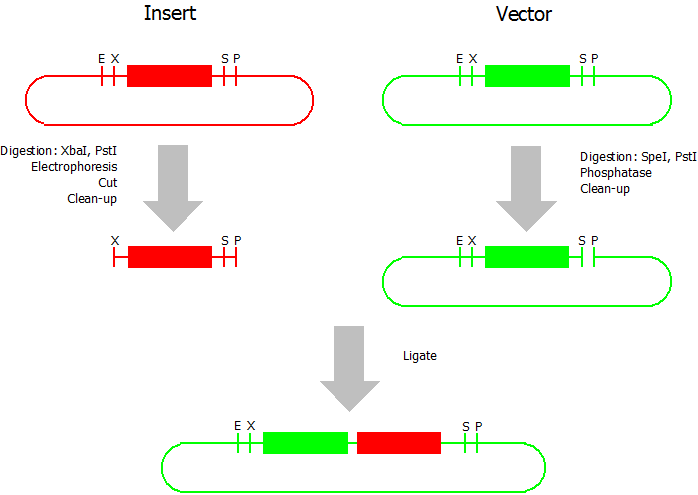
This assembly method can be used for BioBricks which are bigger than 150 bp. The BioBrick should be at least 500 bp bigger or smaller than the backbone. The BioBrick, which complies with these conditions, is used as the insert and is assembled into the prefix or suffix of the other used BioBrick, called vector. So you have to differentiate between a prefix and a suffix insertion.
Suffix Insertion
- Digestion of insert: at least 700 ng DNA / 10 µL volume, 1 µL 10x Tango buffer, 0.5 µL XbaI, 1 µL PstI. Digest for 2 h at 37 °C, afterwards inactivation for 20 min at 80 °C. Clean up the insert via gel electrophoresis. When cutting the insert out of the gel try to avoid staining or exposure to ultraviolet light of the insert.
- Digestion of vector about 700 ng DNA / 10 µL volume, 1 µL 10x orange buffer, 0.5 µL SpeI, 0.5 µL PstI. Digest for 2 h at 37 °C, afterwards inactivation for 20 min at 80 °C. Add 1 µL SAP (shrimp alcaline phosphatase) and 1.2 µL 10 x SAP buffer, incubate for 1 h at 37 °C. Clean up the vector with a PCR clean-up kit.
- Ligation: after digestion and clean-up: 50 - 200 ng of vector, 3 - 10 fold molar access of insert, 20 µL ligation volume, 2 µL T4-Ligase-Buffer, 1 µL T4-Ligase. Incubate for 20 - 30 min at room temperature, afterwards inactivation for 5 min at 70 °C. Then: store at -20 °C or transform.
Prefix Insertion
- Digestion of insert: at least 700 ng DNA / 10 µL volume, 1 µL 10x BamHI buffer, 0.5 µL EcoRI, 0.5 µL SpeI. Digest for 2 h at 37 °C, afterwards inactivation for 20 min at 80 °C. Clean up the insert via gel electrophoresis. When cutting the insert out of the gel try to avoid staining or exposure to ultraviolet light of the insert.
- Digestion of vector about 700 ng DNA / 10 µL volume, 1 µL 10 x Tango buffer, 0.5 µL EcoRI, 0.5 µL XbaI. Digest for 2h at 37 °C, afterwards inactivation for 20 min at 80 °C. Add 1 µL SAP (shrimp alcaline phosphatase) and 1.2 µL 10 x SAP buffer, incubate for 1 h at 37 °C. Clean up the vector with a PCR clean-up kit.
- Ligation: after digestion and clean-up: 50 - 200 ng of vector, 3 - 10 fold molar access of insert, 20 µL ligation volume, 2 µL T4-Ligase-Buffer, 1 µL T4-Ligase. Incubate for 20 - 30 min at room temperature, afterwards inactivation for 5 min at 70 °C. Then: store at -20 °C or transform.
Variations
- A digestion over night is possible. If you digest over night use only 0.1 µL restriction enzyme.
- It is also possible to use PCR product as insert. Digest after PCR with corresponding restriction enzymes and clean up with PCR clean-up kit. This could lead to higher yields of insert DNA because a lot of DNA gets lost during the gel electrophoresis clean up.
- Sometimes some BioBricks are hard to assemble. Then you have to clean up the vector by gel electrophoresis as well.
Standard Freiburg BioBrick Assembly
modified from [http://openwetware.org/wiki/Silver:_BB_Strategy Silver lab] and [http://partsregistry.org/Assembly_standard_25 Assembly standard 25]:
This assembly method can be used for fusion protein assemblies with BioBricks which are bigger than 150 bp. The BioBrick should be at least 500 bp bigger or smaller than the backbone. The BioBrick, which complies with these conditions, is used as the insert and is assembled into the prefix or suffix of the other used BioBrick, which is called vector and needs to be available in the BioBrick [http://partsregistry.org/Assembly_standard_25 Assembly standard 25]. You have to differentiate between a prefix and a suffix insertion.
Suffix Insertion
- Digestion of insert: at least 700 ng DNA / 10 µL volume, 1 µL 10x NEB buffer 4 + 0.1 µL 100x BSA, 0.5 µL NgoMIV (NEB), 1 µL PstI. Digest for 2 h at 37 °C, afterwards inactivation for 20 min at 80 °C. Clean up the insert via gel electrophoresis. When cutting the insert out of the gel try to avoid staining or exposure to ultraviolet light of the insert.
- Digestion of vector about 700 ng DNA / 10 µL volume, 1 µL 10x orange buffer, 0.5 µL AgeI, 0.5 µL PstI. Digest for 2 h at 37 °C, afterwards inactivation for 20 min at 80 °C. Add 1 µL SAP (shrimp alcaline phosphatase) and 1.2 µL 10x SAP buffer, incubate for 1 h at 37 °C. Clean up the vector with a PCR clean-up kit.
- Ligation: after digestion and clean-up: 50 - 200 ng of vector, 3 - 10 fold molar access of insert, 20 µL ligation volume, 2 µL T4-Ligase-Buffer, 1 µL T4-Ligase. Incubate for 20 - 30 min at room temperature, afterwards inactivation for 5 min at 70 °C. Then: store at -20 °C or transform.
Prefix Insertion
- Digestion of insert: at least 700 ng DNA / 10 µL volume, 1 µL 10x orange buffer, 0.5 µL EcoRI, 0.5 µL AgeI. Digest for 2 h at 37 °C, afterwards inactivation for 20 min at 80 °C. Clean up the insert via gel electrophoresis. When cutting the insert out of the gel try to avoid staining or exposure to ultraviolet light of the insert.
- Digestion of vector about 700 ng DNA / 10 µL volume, 1 µL 10 x NEB buffer 4, 0.5 µL EcoRI, 0.5 µL NgoMIV (NEB). Digest for 2h at 37 °C, afterwards inactivation for 20 min at 80 °C. Add 1 µL SAP (shrimp alcaline phosphatase) and 1.2 µL 10 x SAP buffer, incubate for 1 h at 37 °C. Clean up the vector with a PCR clean-up kit.
- Ligation: after digestion and clean-up: 50 - 200 ng of vector, 3 - 10 fold molar access of insert, 20 µL ligation volume, 2 µL T4-Ligase-Buffer, 1 µL T4-Ligase. Incubate for 20 - 30 min at room temperature, afterwards inactivation for 5 min at 70 °C. Then: store at -20 °C or transform.
Variations
- A digestion over night is possible. If you digest over night use only 0.1 µL restriction enzyme.
- It is also possible to use PCR product as insert. Digest after PCR with corresponding restriction enzymes and clean up with PCR clean-up kit. This could lead to higher yields of insert DNA because a lot of DNA gets lost during the gel electrophoresis clean up.
- Sometimes some BioBricks are hard to assemble. Then you have to clean up the vector by gel electrophoresis as well.
Standard 3A assembly
Modified from [http://ginkgobioworks.com/support/BioBrick_Assembly_Manual.pdf BioBrick Assembly Manual by Ginkgo BioWorks]
Digestion
- Thaw DNA from upstream and downstream part and the destination plasmid on ice.
- Destination plasmid has to carry the ccdB gene <partinfo>P1010</partinfo> as insert and has to have a different antibiotic resistance than the plasmids carrying the upstream and downstream parts
- DNA has to be cleaned (by MiniPrep or after a PCR)
- 500 ng DNA / digestion mix for upstream part, downstream part and destination plasmid (total volume of mix 10 µL, dilute with ddH20 if necessary)
- Add 1 µL of 10x buffer and restriction enzymes as shown in the following table:
| Upstream part | Downstream part | Destination plasmid | |
|---|---|---|---|
| enzyme 1 | 0.5 µL EcoRI | 0.5 µL XbaI | 0.5 µL EcoRI |
| enzyme 2 | 0.5 µL SpeI | 1 µL PstI | 0.5 µL PstI |
| buffer | BamHI | Tango | Orange |
- Incubation of the digestion mixes at 37 °C
- After 2 h: add 0.5 µL SAP (shrimp alcaline phosphatase) and 1.15 µL 10x SAP buffer to destination plasmid mix, continue incubation at 37 °C
- After another hour: heat inactivation of all mixes for 20 min at 80 °C
- Continue with ligation or freeze the mixes
Ligation
- Ligation mix:
- 2 µL ddH2O
- 5 µL of every digestion mix (so 15 µL in total)
- 2 µL T4-DNA-ligase buffer (thaw on ice!)
- 1 µL T4-DNA-ligase
- Incubate at least 20 min at room temperature, afterwards heat inactivation for 5 min at 70 °C (optional)
- Freeze ligation mix or continue with transformation (heatshock or electroporation)
Freiburg 3A assembly
Modified from [http://ginkgobioworks.com/support/BioBrick_Assembly_Manual.pdf BioBrick Assembly Manual by Ginkgo BioWorks] and [http://partsregistry.org/Assembly_standard_25 Assembly standard 25]
Digestion
- Thaw DNA from upstream and downstream part (=N-terminal and C-terminal protein domain) and the destination plasmid on ice.
- Destination plasmid has to carry the ccdB gene <partinfo>P1010</partinfo> as insert and has to have a different antibiotic resistance than the plasmids carrying the upstream and downstream parts
- DNA has to be cleaned (by MiniPrep or after a PCR)
- 500 ng DNA / digestion mix for upstream part, downstream part and destination plasmid (total volume of mix 10 µL, dilute with ddH20 if necessary)
- Add 1 µL of 10x buffer and restriction enzymes as shown in the following table:
| Upstream part | Downstream part | Destination plasmid | |
|---|---|---|---|
| enzyme 1 | 0.5 µL EcoRI | 0.5 µL NgoMIV | 0.5 µL EcoRI |
| enzyme 2 | 0.5 µL AgeI | 1 µL PstI | 0.5 µL PstI |
| buffer | Orange | NEB buffer 4 + BSA | Orange |
- Incubation of the digestion mixes at 37 °C
- After 2 h: add 0.5 µL SAP (shrimp alcaline phosphatase) and 1.15 µL 10x SAP buffer to destination plasmid mix, continue incubation at 37 °C
- After another hour: heat inactivation of all mixes for 20 min at 80 °C
- Continue with ligation or freeze the mixes
Ligation
- Ligation mix:
- 2 µL ddH2O
- 5 µL of every digestion mix (so 15 µL in total)
- 2 µL T4-DNA-ligase buffer (thaw on ice!)
- 1 µL T4-DNA-ligase
- Incubate at least 20 min at room temperature, afterwards heat inactivation for 5 min at 70 °C (optional)
- Freeze ligation mix or continue with transformation (heatshock or electroporation)
Restriction analysis
- Digest BioBrick of interest: about 400 ng DNA / 10 µL volume, 1 µL 10x orange buffer, 0.5 µL NotI or PstI. Digest for 2 h at 37 °C. NotI is used to determine the length of the BioBrick and the plasmid backbone, PstI ist used to determine the length of the BioBrick in the plasmid backbone.
- Gel electrophoresis: add 2 µL loading buffer to every digestion mix, apply about 100 - 200 ng DNA / pocket in gel. Don't forget to apply the uncut BioBrick as well. A good agarose concentration for BioBricks between 0.2 and 3 kb is 1.5 %. The smaller your BioBrick of interest is the higher the agarose concentration should be and vice versa. The gel electrophoresis is made with TAE-buffer. Be sure that you melt your agarose gel in the same buffer you use for the electrophoresis later.
Colony PCR
- Pick one colony with a sterile tip and elute it in 100 µL ddH20 or medium
- Store the colony in 4 °C while colony PCR is running
- One reaction mix contains:
- 10 µL 5x buffer
- 2 µL MgCl2 (25 mM stock)
- 1 µL dNTPs
- 0.5 µL primer mix (prefix/suffix primers or sequencing primers)
- 35.25 µL ddH2O
- 0.25 µL GoTaq polymerase (Promega)
- 1 µL template
- PCR program:
- Start: 3 min, 98 °C
- 30 cycles of:
- 30 s, 98 °C
- 30 s, 55 °C
- 30 s / 1 kb template, 72 °C
- Finish: 5 min, 72 °C
- Gel electrophoresis: check the fragment size
- Plate the correct colony
Bisphenol A analysis
Extraction with ethylacetate
- mix 100 µL culture supernatant with 100 µL internal standard (bisphenol F, 100 µg L-1)
- add 200 µL ethylacetate (HPLC grade) for extraction
- vortex (30 s)
- centrifuge for phase separation (5 min, 5000 g)
- take a bit from upper phase and put it in a clean eppi
- SpeedVac at 40 °C to remove ethlyacetate
- solve remaining BPA in water (HPLC grade), vortex (30 s)
- solubility of BPA in water only 300 mg L-1
- for LC-MS analysis of BPA, 300 mg BPA L-1 is definitely enough
- if you want to detect or expect higher concentrations of BPA, solve it in an acetonitrile-water-mix
HPLC method
- C18 reverse phase column
- gradient starting with 45 % acetonitrile up to 95 % acetonitrile
NAD+ bioassay
For the design of Molecular Beacons different [http://www.molecular-beacons.com/MB_SC_design.html tools] might help you out so that a correct function is ensured under defined assay conditions. All measurements were done in the same assay buffer which was composed as follows:
- 50 mM Tris-HCl, pH = 8.0
- 10 mM MgCl2
- 2,5 mM CaCl2
- 5 mM DTT
- 0,05 % BSA
Characterisation of Molecular Beacons
- Thermal profile analysis:
- Prepare a 30 µL reaction mix out of Molecular Beacon buffer and 10 µM Molecular Beacons.
- Signal to background ratio:
- Imaging:
Purification of DNA Ligase
NAD+ detection
Used enzymes
| Enzyme | Producer |
|---|---|
| GoTaq DNA-polymerase | Promega |
| KOD Hotstart DNA-polymerase | Novagen |
| NgoMIV | NEB |
| OneTaq DNA-polymerase | NEB |
| Pfu DNA-polymerase | Promega |
| Phusion HF DNA-polymerase | Finnzymes |
| Restriction enzymes (except NgoMIV) | Fermentas |
| Shrimp alcaline phosphatase | Fermentas |
| T4-DNA-Ligase | Fermentas |
| taq DNA-polymerase | Bioline |
Used Kits
| Function | Name |
|---|---|
| Plasmid purification | Fermentas GeneJET™ Plasmid Miniprep Kit |
| Plasmid purification | Promega PureYield™ Plasmid Preps |
| PCR Cleanup | Macherey Nagel NucleoSpin® Extract II |
| PCR Cleanup | Promega Wizard® SV Gel and PCR Clean-Up |
Media, buffer, solutions etc.
TAE buffer
For 1 L of 50 x TAE buffer you need:
- 242.48 g Tris
- 41.02 g Sodiumacetate
- 18.612 g EDTA
- Adjust pH to 7.8 with acetic acid
- Solve in dH2O
10 mL of the stock is diluted in 1 L dH2O for the gel electrophoresis (0.5 x TAE buffer).
DNA loading buffer
- 50 % (v/v) glycerol
- 1 mM EDTA
- 0.1 % (w/v) bromphenol blue
- Solve in ddH20
LB medium
For 1 L of LB medium you need:
- 10 g Trypton
- 5 g yeast extract
- 10 g NaCl
- 12 g Agar-Agar (for plates)
- Adjust pH to 7.4
Used chemicals
| Chemical | Producer | Purity |
|---|---|---|
| Acetonitrile | VWR | 99.9 %, HPLC Grade |
| Bisphenol F | [http://www.alfa.com/de/GP100W.pgm?DSSTK=A11417&rnd=097380668 Alfa Aesar] | 98 % |
| Ethylacetate | VWR | > 99.5 %, p.a. |
| Chemical A | [http://www.link.com Producer A] | XX.X % |
 "
"