July 24
Started overnight cultures of pSB3K3
July 25
PCR'd parts for pNT002: R0040, K123001, B0014, pSB4A5
PCR'd GFP control insert and pNT003 insert
Gibson assembly and transformation of pNT003 with a negative control
Nanodrop of evaporation concentrated PFGE DNA: 9-1: 11.4ng/uL; 10-2: 29.7ng/uL
Attempt at packaging above DNA with fosmid kit extract, but enzyme wasn't heat inactivated as par procedure
50 mL of phage dillution buffer made for fosmid kit
Evaporation concentrated more PFGE DNA. Nandrop results: 9mix: 14.3ng/uL; 10mix: 2.2ng/uL
Overnight ligation of 9mix and (10mix + 10-2) to redo fosmid packaging, at 16°C
Results
Decided not to use the pNT003 PCR'd insert, as the band of the correct length was much fainter than a lower length band. Chose not to do Gibson assembly of the positive GFP control, despite not having any green colonies last week, instead focusing on assembling pNT002 and pNT003.
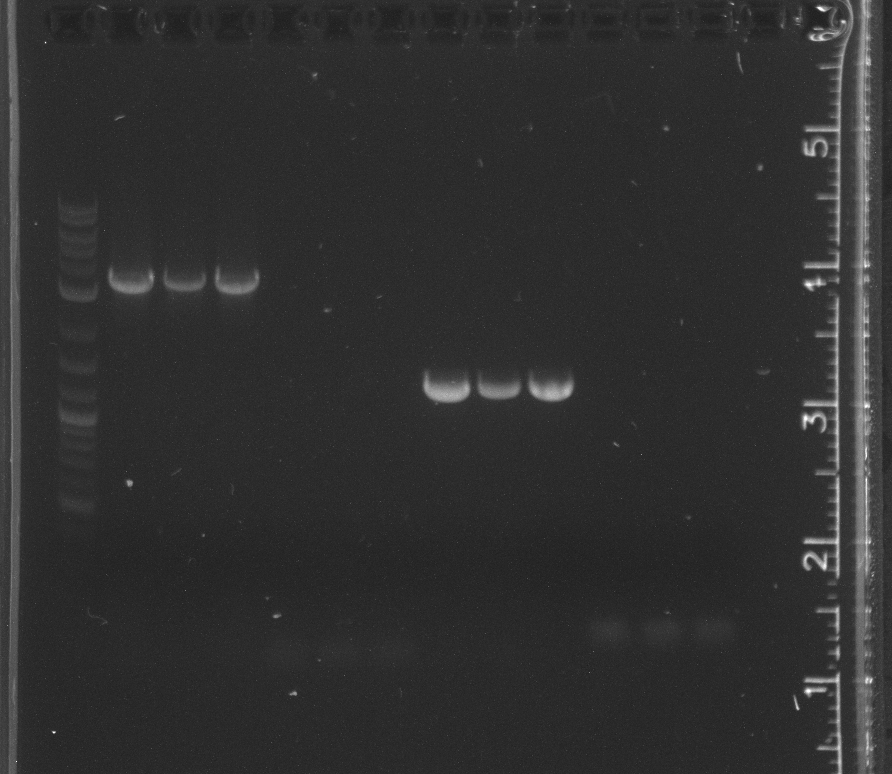
lane 1 NEB 2-log ladder, 2-4 pSB4A5, 5-7 R0040, 8-10 K123001, 11-13 B0014

lane 1 NEB 2-log ladder, 2-4 pNT003 insert, 5 R0010, 6 K123003, 7 B0014, 8 blank, 9-11 GFP insert, 12 GFP constitutive promoter, 13 GFP, 14 GFP terminator, 15 NEB 2-log ladder
PCR concentrations
| Part |
Concentration (ng/ul) |
| R0040 for pNT002 |
56.4 |
| K123001 |
121.5 |
| B0014 for pNT002 |
100.8 |
| pSB4A5-1 |
98.0 |
| pSB4A5-2 |
97.4 |
July 26
Packaging of 9mix and (10mix + 10-2) ligations
Amanda did something
Attempted various methods of spreading chemical solution on minimal media plates
16s PCR
July 27
Grew up cells for titering of packaged fosmids-Reached OD600 of 0.974
16s PCR gel from yesterday had bands in negative control
Redid 16s PCR
Amanda did something
Julia did something
Minimal media transfers
July 28
Plate packaged fosmids to obtain titer
Set up enzyme binding assay of p450s
Try out plating chemicals on bottom of plates with agar bacteria suspension on top
Try conventional assembly of pNT002 and pNT003
Find positive control for transformation, test transformation
Run gel of 16s, DpnI digest, purify, gibson
 "
"


