July, 4th
Digestions of previously purified plasmids were performed for ligations:
| Plasmid |
Kind |
Purified DNA (μl) |
H2O (μl) |
Enzyme 1 (μl) |
Enzyme 2 (μl) |
Buffer H (μl) |
Final Volume(μl) |
| <partinfo>BBa_C0060</partinfo> |
Insert |
16.5 |
4 |
1 EcoRI |
1 SpeI |
2.5 |
25 |
| <partinfo>BBa_C0061</partinfo> |
Insert |
13.2 |
7.3 |
1 XbaI |
1 PstI |
2.5 |
25 |
| <partinfo>BBa_K081022</partinfo> |
Insert |
15.7 |
4.8 |
1 EcoRI |
1 PstI |
2.5 |
25 |
| <partinfo>BBa_B0030</partinfo> |
Vector |
13.2 |
7.3 |
1 SpeI |
1 PstI |
2.5 |
25 |
| <partinfo>BBa_B0031</partinfo> |
Vector |
12.4 |
8.1 |
1 SpeI |
1 PstI |
2.5 |
25 |
| <partinfo>BBa_B0032</partinfo> |
Vector |
9.5 |
11 |
1 SpeI |
1 PstI |
2.5 |
25 |
| <partinfo>BBa_B0015</partinfo> |
Vector |
7.9 |
12.6 |
1 EcoRI |
1 XbaI |
2.5 |
25 |
| <partinfo>pSB4C5</partinfo> |
Vector |
3 |
17.5 |
1 EcoRI |
1 PstI |
2.5 |
25 |
| <partinfo>BBa_I13501</partinfo> |
Insert |
7.2 |
13.3 |
1 XbaI |
1 PstI |
2.5 |
25 |
Reactions were incubated at 37°C for three hours while a small-size and a medium-size agarose gel were prepared according to protocols.
In the afternoon gel electrophoresis was performed.
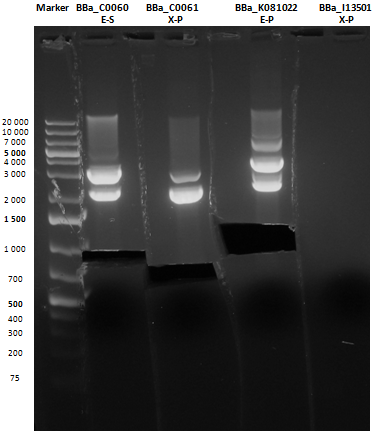 Small size gel |
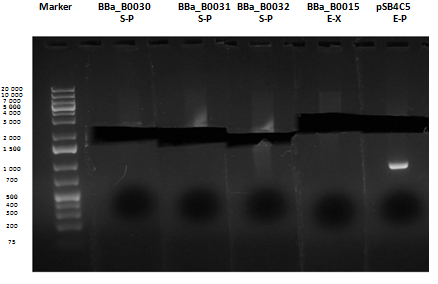 Medium size gel |
As shown, in figure all clones were positive (apart from <partinfo>BBa_I13501</partinfo> run where we inexplicably didn't see any band), so we cut and purified the bands of interest. Cells harbouring <partinfo>BBa_I13501</partinfo> were again inoculated in 5 ml LB + Amp.
After gel extraction, cut DNA was quantified:
| Plasmid |
Purified DNA ng/μl |
| <partinfo>BBa_C0060</partinfo> (E-S) |
3.9 |
| <partinfo>BBa_C0061</partinfo> (X-P) |
4.1 |
| <partinfo>BBa_K081022</partinfo> (E-P) |
4.4 |
| <partinfo>BBa_B0030</partinfo> (S-P) |
10.3 |
| <partinfo>BBa_B0031</partinfo> (S-P) |
11.8 |
| <partinfo>BBa_B0032</partinfo> (S-P) |
10.1 |
| <partinfo>BBa_B0015</partinfo> (E-X) |
12 |
| <partinfo>pSB4C5</partinfo> (E-P) |
10.7 |
Then ligations were performed in a final volume of 10 μl:
| Ligation Name |
Vector |
Vector μl |
Insert |
Insert μl |
Buffer μl |
T4 Ligase μl |
| E1 |
<partinfo>BBa_B0015</partinfo> (E-X) |
1.5 |
<partinfo>BBa_C0060</partinfo> (E-S) |
6.5 |
1 |
1 |
| E2 |
<partinfo>BBa_B0030</partinfo> (S-P) |
1.5 |
<partinfo>BBa_C0061</partinfo> (X-P) |
6.5 |
1 |
1 |
| E3 |
<partinfo>BBa_B0031</partinfo> (S-P) |
1.5 |
<partinfo>BBa_C0061</partinfo> (X-P) |
6.5 |
1 |
1 |
| E4 |
<partinfo>BBa_B0032</partinfo> (S-P) |
1.5 |
<partinfo>BBa_C0061</partinfo> (X-P) |
6.5 |
1 |
1 |
| E8 |
<partinfo>pSB4C5</partinfo> (E-P) |
1.5 |
<partinfo>BBa_K081022</partinfo> (E-P) |
6.5 |
1 |
1 |
Reactions were incubated ON at 16°C.
 "
"

